BIO254:DarkNoise
Introduction
Noise is defined as "an unwanted signal or a disturbance in an electronic device or instrument; broadly, it is a disturbance interfering with the operation of a usually mechanical device or system". (from Merriam-Webster Online Dictionary)
Our ability to detect dim lights is limited by noise in the rod photoreceptors. These electrical events prduced in the dark which is indistinguishable from the real signal produced by light especially limit visual sensitivity at low levels of illumination. In the 1942 psychophysical experiments of Hecht, Shlaer, and Pirenne, it is showed that our dark-adapted viusal system can successfully detect the absorption of 5-7 photons. More recent reserach indicates that the ultimate limit on the accuracy of photon counting is imposed by dark noise in the retinal rods.
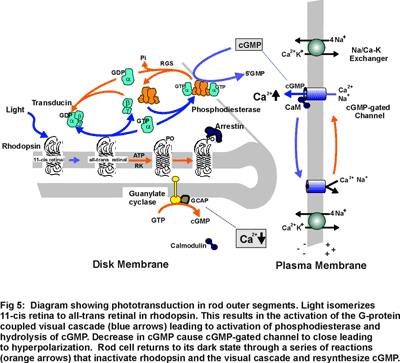
Both rods and cones photoreceptors respond to light by hyperpolarizing the membrane potential. This dark noise signal affects the synapse of photoreceptors only when it produced a change in membrane current that exceed a certain threshold. This threshold is a fundamental characteristic of the phototransducion process, defined as the amplitude of the spontaneous membrane current in the dark, is referred as the dark noise.
Two components of electrical dark noise
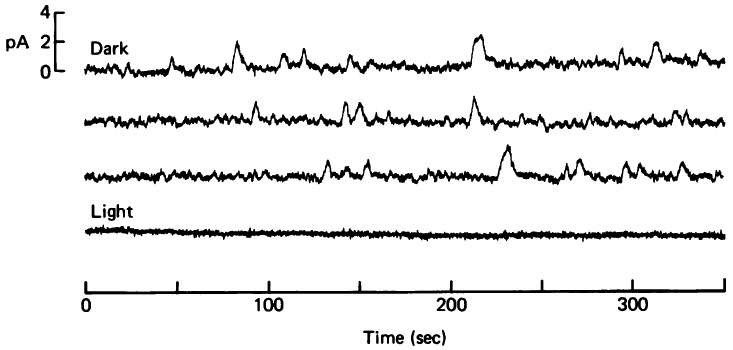
Researchers (Simon, Lamb & Hodgkin, 1975; Schwartz 1977) first reported a small random fluctuations of the membrane potential of vertebrate photoreceptors in the darkness, this so-called dark noise lessens during the response to bright steady light. Later, Baylor DA et al study this physiological noise in the visual transduction mechanism by recording membrane current from single rod outer segments in pieces of isolated toad retina, they used the suction pipette technique (see below) to examine the components of the dark noie. They found that the inward current detected in darkness showed spontaneous fluctuations which disappeared during the response to bright light. They also found that the dark noise has two components: "a continuous fluctuation of rms amplitude about 0.2pA and occational dicrete events about 1pA in size". (J. Physiol. (1980), 309, pp. 591-621)
Discrete noise
The discrete noises are rarer and larger, compared to continuous noise. The amplitude and power spectrum of the discrete events resembled those of single photon effects in the same rod. This suggests that discrete noise may arise from spontaneous activation of single rhodopsin molecules. Besides, the intervals between these occational discrete events followed the exponential distribution expected of a Poisson process with a mean rate of about one event per 50 sec (20 oC). Also, the temperature dependence of the mean frequency of occurrence of discrete events has an activation energy of 22 kcal mole-1, probably is characteristic of thermal isomerization of rhodotin. In fact, the the spontaneous isomerization of 11-cis retinal in rhodopsing is so rare (ie. on average, an isomerization time for a rhodopsin molecule is 3000 years, it doesn't happen often), makes rods capable of detecting single phtotons.
Continuous noise
The variance of the continuous noise rose linearly with the length of the outer segment drawn into the suction electord, indicating that this component is generated in the outer segment. Furthermore, the continuous noise persists when a rod is voltage-clamped, indicating that the noise arises from fluctuations in outer-segment conductance rather than fluctuations in the driving potential on the outer-segment current. Also, the frequency composition of the continuous noise can be predicted from the kinetics of the cells's response to a dim flash, suggesting continuous noise arises in the transduction cascade downstream from rhodopsin. Unlike discrete noise, the molecular mechanism of the continuous components is not known until 1996. This year, F. Rieke and D.A. Baylor investigate it in toad rods. They record the membrane current from intact and isolated rods and truncated, internally dialyzed rod outer segments. They seperate continuous noise from other noise. By selectively disablzing different elements of the phototransduction cascade, they examine their contributions to the continuous noise. Their experiments indicate that this noise is generated by spontaneous activation of cGMP phosphodiesterase (PDE) through a process that does not involve transducin.
Dark noise in rods and cones
It has been been demonstrated that cones have more noise than rods in the absence of any light. (Lamb and Simon. 1977; Schnapf et al., 1990; Schneeweis and Schnapf, 1999) For example, in primate, the amplitude of the noise is about 0.12pA in cones and is 0.03 pA in rods. Hence, rods is more sensitive than cones.
In darkness, primate rods give occasional spontaneous signal resembling reponses to single photon, just like toad. This photon-like dark noise in primate rods may result from thermal isomerization of rhodopsin. Under this assumption, plus the number of rhodopsin in a rod and rate of the noise events, the half-life of the thermal decay is 420 years. This greate stability of rhodopsin in darkness allows primate (us) to have reliable detection of very dim light.
Neither the molecular origin of the cone noise nor its functional implications are known until later the Neuron paper of 2000, "Origin and Functional Impact of Dark Noise in Retinal Cones." In this paper, they show the origin of the dark noise in salamander cones varies with cone type. Details could be found on this paper.
Major technique used in dark noise
Before the suction pipette techniques was developd in 1977, the light response of photoreceptors can only be studied by intracellular recording or by measuring extracelular voltage gradients. The disadvantage of both models is that both models can only provide information averaged over many photoreceptors.
Suction pipette techniques: To fix the problem mentioned above, and to record the elementary events of one photoreceptor, Baylor et al developed a new technique to record the membrane current of a single rod outer segment. Here is how it works: first, small pieces of retina were isolated from dark-adapted toad and kept in oxygenated toad Ringer. By using an inverted microscope and infrared image converter, they draw a single rod outer segment and fit it into a close-fitting suction electrode containing Ringer. A current sensor between the pipette and a reference electrode in a bathing solution recorded the bulk of membrane current flowing through the region of the outer segment of the rod within the pipette. Finally, a transverse slit of light (or no light=dark) is applied to the outer segment from an optical stimulator.
Major reference of this techniques:
YAU, K.-W., LAMB, T.D. & BAYLOR, D. A. (1977)
Lght-induced fluctuations in membrane current of signle toad rod outer segments. Nature. Lond. 269, 78-80
McBURNEY, R.N. & NORMANN, R.A. (1977)
Current and voltage responses from single rods in toad retina. J. Gen. Physiol. 70, 12a
References
Baylor, D. A., Matthews, G., and Yau, K.-W., (1980) J. Physiol. 309, 591-621
Hescht, Shlaer, and Pirenna, (1942) J. Gen. Physiol. 25, 819
Schwartz, E. A. J. Physiol. 272, 217-246
Simon, E. J., Lamb, T.D. & Hodgkin, A.L. (1975) Nature. Lond. 256, 661-662.
Baylor, D. A. (1987) Investigative ophthalmology & visual Science. Proctor Lecture Vol 28. 34-50.
Schnapf, J. L., Baylor, D. A. (1984) J. Physiol 357. pp. 575-602
Rieke, F., Baylor, D. A. (2000) Neuron. 26, 181-186
Rieke, F., Baylor, D. A. (1996) Biophys. J. 71, 2553-2572
Useful links
http://webvision.med.utah.edu/ link title
Recent updates to the site:
- N
- This edit created a new page (also see list of new pages)
- m
- This is a minor edit
- b
- This edit was performed by a bot
- (±123)
- The page size changed by this number of bytes
3 May 2024
| 15:02 | CHIP:Talks diffhist 0 Gabor Balazsi talk contribs | ||||
| 15:01 | CHIP:Data diffhist −23 Gabor Balazsi talk contribs | ||||
|
|
14:33 | UA Biophysics:Protocols:Kanamycin 5 changes history −421 [Elizabeth Suesca (5×)] | |||
|
|
14:33 (cur | prev) +13 Elizabeth Suesca talk contribs | ||||
|
|
14:21 (cur | prev) +16 Elizabeth Suesca talk contribs (→Materials) | ||||
|
|
14:19 (cur | prev) −6 Elizabeth Suesca talk contribs | ||||
|
|
14:14 (cur | prev) −50 Elizabeth Suesca talk contribs | ||||
|
|
14:13 (cur | prev) −394 Elizabeth Suesca talk contribs | ||||
|
|
14:33 | UA Biophysics:Protocols:Ampicillin 4 changes history −379 [Elizabeth Suesca (4×)] | |||
|
|
14:33 (cur | prev) +15 Elizabeth Suesca talk contribs | ||||
|
|
14:19 (cur | prev) −7 Elizabeth Suesca talk contribs | ||||
|
|
14:11 (cur | prev) 0 Elizabeth Suesca talk contribs (→PROTOCOL) | ||||
|
|
14:09 (cur | prev) −387 Elizabeth Suesca talk contribs | ||||
|
|
14:31 | UA Biophysics:Protocols:Erythromycin 3 changes history −315 [Elizabeth Suesca (3×)] | |||
|
|
14:31 (cur | prev) −7 Elizabeth Suesca talk contribs | ||||
|
|
14:30 (cur | prev) −12 Elizabeth Suesca talk contribs (→Materials) | ||||
|
|
14:18 (cur | prev) −296 Elizabeth Suesca talk contribs | ||||
|
|
14:29 | UA Biophysics:Protocols:Chloramphenicol 2 changes history −427 [Elizabeth Suesca (2×)] | |||
|
|
14:29 (cur | prev) +36 Elizabeth Suesca talk contribs | ||||
|
|
14:16 (cur | prev) −463 Elizabeth Suesca talk contribs | ||||
|
|
N 12:09 | BioMicroCenter:Oligo Synthesis 7 changes history +4,408 [Noelani Kamelamela (7×)] | |||
|
|
12:09 (cur | prev) −14 Noelani Kamelamela talk contribs (→Dr Oligo 96) | ||||
|
|
12:09 (cur | prev) −16 Noelani Kamelamela talk contribs (→STX-200) | ||||
|
|
12:08 (cur | prev) −46 Noelani Kamelamela talk contribs (→Dr Oligo 96) | ||||
|
|
12:08 (cur | prev) +35 Noelani Kamelamela talk contribs (→Dr Oligo 96) | ||||
|
|
08:29 (cur | prev) +7 Noelani Kamelamela talk contribs (→Dr Oligo 96) | ||||
|
|
08:27 (cur | prev) +59 Noelani Kamelamela talk contribs (→Dr Oligo 96) | ||||
| N |
|
08:16 (cur | prev) +4,383 Noelani Kamelamela talk contribs (Created page with "{{BioMicroCenter}} ''' ** All users must be trained before being allowed to use the equipment **'''<BR><BR> The BioMicro Center currently hosts two oligo synthesizers: one Dr Oligo 96 (Biolytic) and one Syntax STX-200 (DNAScript). <br><br> Dr Oligo is optimized for higher concentrations of oligos made through polyamidite synthesis. For ready to use oligos a series of steps must be completed using the other related machines in the Center including the column presser, the...") | |||
|
|
12:06 | BioMicroCenter:Covaris 5 changes history +172 [Noelani Kamelamela (5×)] | |||
|
|
12:06 (cur | prev) −5 Noelani Kamelamela talk contribs (→R230) | ||||
|
|
09:55 (cur | prev) +2 Noelani Kamelamela talk contribs (→R230) | ||||
|
|
09:54 (cur | prev) +1 Noelani Kamelamela talk contribs (→R230) | ||||
|
|
09:52 (cur | prev) +4 Noelani Kamelamela talk contribs (→R230) | ||||
|
|
08:41 (cur | prev) +170 Noelani Kamelamela talk contribs (→R230) | ||||
|
|
09:52 | (Upload log) [Noelani Kamelamela (6×)] | |||
|
|
09:52 Noelani Kamelamela talk contribs uploaded File:R230pic.jpg | ||||
|
|
08:42 Noelani Kamelamela talk contribs uploaded File:R230pic.jpeg | ||||
|
|
08:32 Noelani Kamelamela talk contribs uploaded File:Syntax.jpg | ||||
|
|
08:31 Noelani Kamelamela talk contribs uploaded File:Droligoprocess.jpg | ||||
|
|
08:29 Noelani Kamelamela talk contribs uploaded File:Chamber.jpg | ||||
|
|
08:28 Noelani Kamelamela talk contribs uploaded File:DrOligo.jpg | ||||
2 May 2024
|
|
19:54 | Paper Microfluidic Device for Archiving Breast Epithelial Cells 10 changes history −572 [Xning098 (10×)] | |||
|
|
19:54 (cur | prev) −10 Xning098 talk contribs (→Diseases tested with neonatal heel pricks) | ||||
|
|
19:53 (cur | prev) −12 Xning098 talk contribs (→Results with Breast Epithelial Cells) | ||||
|
|
19:49 (cur | prev) 0 Xning098 talk contribs (→Current diagnostic tools) | ||||
|
|
19:45 (cur | prev) +45 Xning098 talk contribs (→Design Parameters) | ||||
|
|
19:40 (cur | prev) −412 Xning098 talk contribs (→Design Parameters) | ||||
|
|
19:35 (cur | prev) 0 Xning098 talk contribs (→Paper Microfluidics) | ||||
|
|
19:32 (cur | prev) 0 Xning098 talk contribs (→Possibilities in Breast Cancer Detection) | ||||
|
|
19:31 (cur | prev) 0 Xning098 talk contribs (→Breast Cancer: Overview) | ||||
|
|
19:30 (cur | prev) −233 Xning098 talk contribs (→Breast Cancer Epigenetics) | ||||
|
|
19:16 (cur | prev) +50 Xning098 talk contribs (→Breast Cancer Epigenetics) | ||||
|
|
14:21 | Biophysics Lab:Reprints 3 changes history +461 [Elizabeth Suesca (3×)] | |||
|
|
14:21 (cur | prev) 0 Elizabeth Suesca talk contribs | ||||
|
|
14:20 (cur | prev) +140 Elizabeth Suesca talk contribs | ||||
|
|
14:13 (cur | prev) +321 Elizabeth Suesca talk contribs | ||||
| 14:04 | UA Biophysics:Protocols diffhist −46 Elizabeth Suesca talk contribs | ||||
| 14:04 | UA Biophysics:Microscopes diffhist −44 Elizabeth Suesca talk contribs | ||||
| 14:04 | UA Biophysics:Equipment diffhist −44 Elizabeth Suesca talk contribs | ||||