Nathan R Beshai Week 3
From OpenWetWare
Jump to navigationJump to search
Nathan R. Beshai User Page
Nathan R. Beshai Template Page
Course assignments
Individual journal assignments
- Nathan R Beshai Week 2
- Nathan R Beshai Week 3
- Nathan R Beshai Week 4
- Nathan R Beshai Week 5
- Nathan R Beshai Week 6
- Nathan R Beshai Week 7
- Nathan R Beshai Week 8
- Nathan R Beshai Week 9
- Nathan R Beshai Week 10
- Nathan R Beshai Week 11
- The D614G Research Group Week 12
- The D614G Research Group Week 14
Class Journals
- Class Journal 1
- Class Journal 2
- Class Journal 3
- Class Journal 4
- Class Journal 5
- Class Journal 6
- Class Journal 7
- Class Journal 8
- Class Journal 9
- Class Journal 10
- Class Journal 11
- Class Journal 12
- Class Journal 14
Link to Brightspace and LMU's Homepage
Purpose
- This journal focuses on being able to correctly interpret scientific articles, be able to outline them, and accurately depict figures within the article.
10 unknown vocabulary and their definitions
- Spike Proteins: "Spike is an envelope glycoprotein which aids viral entry into the host cell" (Interpro, Classification of protein families).
- Angiotensin converting enzyme: "this hydrolase enzyme cleaves the decapeptide angiotensin i (biologically inactive) to form active angiotensin ii by angiotensin-converting enzyme which removes a dipeptide (histidylleucine) from angiotensin i" (Biology online).
- Animal reservoir: " The reservoir typically harbors the infectious agent without injury to itself and serves as a source from which other individuals can be infected" (MedicineNet).
- Palm Civet: "any of various small to medium-sized, chiefly arboreal cats of the civet family, of southeastern Asia, the East Indies, etc., with a spotted or striped coat and a long curled tail" (Dictionary.com)
- Salt Bridge: "are elementary motifs of protein secondary and tertiary structure and are commonly associated with structural driving force that increases stability" (Pylaeva et al. 2018).
- MERS: ": a serious viral respiratory illness that is marked by fever, cough, and shortness of breath and that may often progress to severe pneumonia with acute respiratory distress syndrome and organ failure" (merriam-webster.com)
- Receptor: "any cellular macromolecule that binds a hormone, neurotransmitter, drug, other agonist, or intracellular messenger to initiate a change in cell" (Oxford University Press).
- Infectious: "1.(of a disease) caused by pathogenic (micro)organisms.2 causing or transmitting infection" (Oxford University Press).
- Peptidase: "any enzyme that hydrolyses peptide bonds. The group includes the exopeptidases, such as the aminopeptidases and carboxypeptidases" (Oxford University Press).
- Residue: "(biochemistry) Any of the monomers comprising a polymer, or any of the parts that integrate to make up a larger molecule" (Biology online).
Outline of the article "Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS Coronavirus"
Abstract
- Covid-19 is a new virus that has spread from Wuhan, China, and causes symptoms that are alike to the SARS-CoV (severe acute respiratory syndrome coronavirus).
- Analysis shows that there is an interaction that occurs between the SARS-CoV protein receptor binding protein and the host receptor angiotensin-converting enzyme 2 (ACE2_.
- The receptor protein regulates the transmission of (SARS-CoV).
- The researchers are checking to see if the 2019-nCov uses the same receptors as SARS-CoV.
- The researchers found four main findings that broadened their knowledge of the "receptor usage, cell entry, host cell infectivity, and animal origin" of the coronavirus epidemic. These findings could aid the surveillance and prevention of Coronavirus.
- The sequence of the coronavirus receptor-binding domain (RDB) and the receptor-binding domain used the ACE2 receptor.
- Residues of the coronavirus's RBM interacted with human ACE2s that were the same for the human cell infection capacity.
- Other residues of the coronavirus's RBM were capable of binding to ACE2 which showed that SARS-COV2 had the ability for interspecies human transmission.
- The bat phylogenetic analysis showed that other animals could act as intermediate hosts.
Importance of the expiriment
- The importance of this experiment is to show the world that SARS-CoV 2 has similarities to the 2002/2003 SARS-CoV outbreak.
- The spike proteins of SARS-CoV and 2019-nCoV use ACE2 as their protein binding receptor, which influences the transmission between humans and animal-human interactions.
- The research in this paper builds off of the SARS-CoV research that was used to prevent and surveillance epidemics, study animal hosts and models of infections, and to study and predict species-specific receptors.
- The main goal is to contribute to the public knowledge of the 2019-nCoV and to aid in the studying and reduction of the virus (Wan et al., page 1).
Introduction
- A new Coronavirus from Wuhan, China has recently presented over 500 cases and more than 17 deaths in China.
- THe 2019-nCoV virus spread and infected people in other countries, such as the United States.
- Like SARS-CoV, the 2019-nCov presents with acute respiratory syndrome symptoms.
- The SARS-CoV infection began in 2002 and ended in 2003 and could transmit between humans.
- Isolated from Palm civets and bats, which were natural hosts.
- This led to about 800 deaths.
- It then reemerged in 2003-2004, however, there were only a couple cases and did not spread among humans.
- It is believed that both viruses were transmitted from Chinese markets, however, the 2019-nCoV virus's source is still unsure.
- Coronaviruses are single-stranded enveloped RNA viruses.
- Single-stranded enveloped RNA viruses have 4 major genera and both SARS-CoV and 2019-nCoV are of the beta-genus.
- The viruses have envelope-anchored spike proteins that enter the cells through binding to the host receptor and fusing the viral and host membranes together.
- The RBD is the domain responsible for identifying the host receptor's ACE2.
- Previous research shows that the host is more likely susceptible to contract SARS-CoV based on how compatible the RBD complex and ACE2 are during the initial attachment phase.
- SARS-CoV has a claw-like ACE2which allows the RBN of the RBNE to bind easily.
- The ACE2 has 2 major binding sites for the RBD.
- The binding site of the ACE2 is located by looking for the RBD mutation residues, showing the host range.
- The RBD also contained identifiable amino acids that increased the likelihood of the viral binding to the Human ACE2.
- Whan all the favorable residues of RBD were present, the spike protein was very efficient in binding to the ACE2.
- The goal of this study was to take the released 2019-nCoV RBD sequences and predict the ACE2 host range and receptor use (Wan et al., page 2).
Results
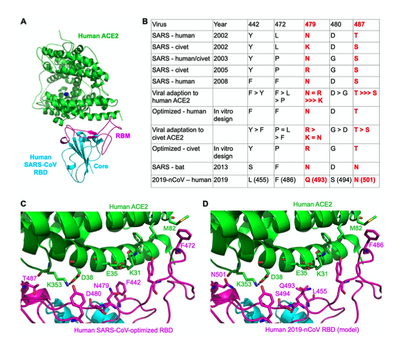
- Figure 1 (Wan et al., page 3)
- Figure 1A. Structural analysis created through software coot and software PyMOL depicting the SARS-CoV RBD and RBM binding to the claw-like ACE2 receptor.
(Wan et al., page 2 and 3). - Figure 1B. Table of the amino acid sequences and mutation residues that were found near the ACE2 receptor hotspots for SARS-CoV and 2019-nCoV. Specifically, it shows 5 residues that play major roles in the RBD protein's binding and transmission (Wan et al., page 3).
- Figure 1C. Structural analysis created through software coot and software PyMOL depicting the 5 amino acid residues of the optimized human SARS-CoV RBD and their binding site on the human ACE2. They were at the amino acid located at the 442 location, 472 location,479 location, 480 location and 487 location on the Human SARS-CoV optimized RBD (Wan et al., page 2 and 3).
- Figure 1d. Structural analysis created through software coot and software PyMOL depicting the 5 amino acid residues of the optimized human 2019-nCoV RBD and their binding site on the human ACE2. They were at the amino acid located at the 493 location (Glutamate), 455 location (Leucine),486 location (phenylalanine), 494 location (Serene), and 501 location (Asparagine) on the Human SARS-CoV optimized RBD (Wan et al., page 3).
- Figure 1A. Structural analysis created through software coot and software PyMOL depicting the SARS-CoV RBD and RBM binding to the claw-like ACE2 receptor.
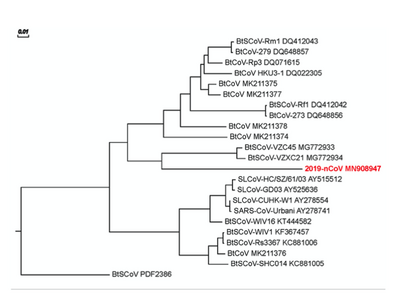
- Figure 2 (Wan et al., page 4)
- Used Geneious prime and the Jukes-Cantor genetic distance model to create a phylogenetic tree of the spike protein sequences of the beta-genus b-lineage coronavirus (Wan et al., page 4 and 8).
- After the sequences were aligned with the BIosum62 cost matrix, the research found that the 2019-nCovspike phylogeny is similar to other beta bonds of the b-bat lineage SARS coronavirus (Wan et al., page 2 and 4).
- Used Geneious prime and the Jukes-Cantor genetic distance model to create a phylogenetic tree of the spike protein sequences of the beta-genus b-lineage coronavirus (Wan et al., page 4 and 8).
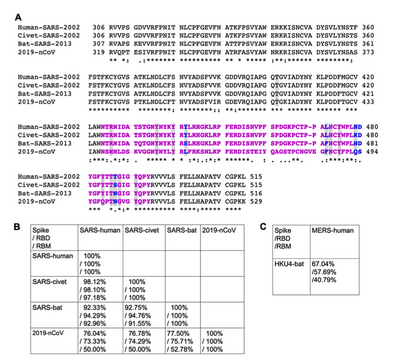
- Figure 3 (Wan et al., page 5)
- Figure 3A. Sequence alignment and comparison of the 2019-nCoV RBM and the SARS-CoV RBM using the program Clustal omega. The colons show the position of strongly conserved residues, the asterisks show single, fully conserved residues, and the periods show weakly conserved residues (Wan et al., page 3 and 5).
- Figure 3B. Shows the comparison of the spike protein of SARS-CoV (Human, civet, and bat strands) and 2019-nCoV RBM and RBDs and demonstrates the sequence similarities (Wan et al., page 5).
- Figure 3C. Shows the comparison of the spike RBD and RBM of the MERS-human and HKU4-bat strands and demonstrates sequence similarities (Wan et al., page 5).
- Figure 3A. Sequence alignment and comparison of the 2019-nCoV RBM and the SARS-CoV RBM using the program Clustal omega. The colons show the position of strongly conserved residues, the asterisks show single, fully conserved residues, and the periods show weakly conserved residues (Wan et al., page 3 and 5).
- Figure 4 (Wan et al., page 6)
- Figure 4A. Shows the "critical changes" in the amino acids of the virus contacting residues in the ACE2 among different species, found from GenBank (Wan et al., page 6).
- Figure 4B. Shows the structural contact points between the ACE2 Residues and the Civet Sars Cov- optimized RBD (Wan et al., page 6).
- Figure 4C. Shows the structural contact points between the ACE2 Residues and the Human 2019-nCoV optimized RBD (Wan et al., page 6 and 7).
Discussion
- Their research aids in finding preventative methods to inhibit the binding of viruses to cell receptors.
- Specifically, their goal of this experiment was to learn about the preventative methods of 2019-nCoV, using their research in SARS-CoV as a foundation.
- Their structural models and sequences showed that the 2019-nCoV virus did bind to the human ACE2 receptor.
- Two other publications agree.
- Unlike SARS-CoV, 2019-nCoV was less efficient in binding to the receptor.
- However, a single mutation in the 2019-nCOV RBD and RBM at the n501 amino acid residue location would cause an increase in the binding of the ACE2 receptor with the RBD.
- The researchers recommend that patients should be monitored for the n501 mutation.
- However, a single mutation in the 2019-nCOV RBD and RBM at the n501 amino acid residue location would cause an increase in the binding of the ACE2 receptor with the RBD.
- The 2019-nCoV virus likely was transmitted from a bat, as it presents a close phylogenetic relationship with other SARS-CoV Beta genus lineages.
- Pigs or Cats are the best study animals to us as models as they have a binding ability for the 2019-nCoV RBD and ACE2 (Wan et al., page 7).
- Their overall goal of the study is to provide public health research communities with more insights to help in the fight against the 2019-nCoV (Wan et al., page 8).
Materials and Methods
- Software Coot induced mutation into structural models and Software PyMOL helped prepare the structural figures.
- Phylogenetic analysis was studied with radial phylograms generated in Geneious prime and used a jukes-cantor genetic distance model.
- The researchers used the neighbor-joining build model with no outgroup and 100 bootstrap replicates.
- The phylogram was rendered in adobe illustrator
- Used Clustal Omega as a protein sequence alignment program (Wan et al., page 8).
Questions not in outline
- What were the goals and results of previous studies that led them to perform this work?
- The previous studies that they referenced wished to create a framework for virus-receptor interactions for future epidemics (Wan et al., 8).
- What future directions should the authors take?
- The authors should study 2019-nCoV samples from across the world and not only from Wuhan databases. The authors stated that if there were any mutations it would change transmission efficiencies. They should check samples across the world to look for mutations that can change the rate of transmission.
- Give a critical evaluation of how well you think the authors supported their conclusions with the data they showed.
- I think the authors did an excellent job given the time that they completed it in and the supplies and access they had. However, I think that they did not show any work that was not from a computer. The models and figures they created were all from databases and software. I believe they should have included lab data to support their work.
- Are there any limitations or major flaws in the paper?
- The limitations in this paper are that the authors did not show what would happen if mutations occurred and transmission rates of the viruses. The authors explained how amino acids affect transmission rates but they did not show examples of what would happen if a mutation occurred. The authors also stated that SARS-CoV was more efficient than 2019-nCoV from 2002-2003 and not as efficient during the limited 2003-2004 but they did not show what changed and did not show how much more or less efficient it was.
Scientific Conclusion
- As biologists it is imperative that a correct interpretation and understanding of scientific articles is needed. When we read and write scientific articles during a crisis, like a coronavirus, getting the information correct for the public is a duty as scientists. When a new disease and virus is present in society, many people may not understand the information being spouted by scientists. Figures and models help the public and even scientists understand the literature better by being able to picture the numerical and physiological aspects of a paper. In this journal, outlining an article, interpreting figures, and defining key words helped break a complex article down and made it easier to clarify the literature.
Acknowlagments
- Referenced and copied OpenWebWare syntax from the BIOL368/F20 week 1 page
- Referenced and copied questions from the BIOL368/F20 week 3 page.
- Referenced the Purdue Owl for aid in citations.
- Referenced the article Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus for the whole journal.
- Worked with my partner Owen Daily on interpreting the figures and assigning roles for the Journal club.
- Referenced the Oxford Dictionary of Biochemistry and Molecular Biology, the Merriam-Webster dictionary, Dictionary.com, Pylaeva et al. 2018, biologyonline.com, MedicineNet.com, and Interpro for definitions.
References
- OpenWetWare. (2020). BIOL368/F20:Week 1. Retrieved September 22, 2020, from https://openwetware.org/wiki/BIOL368/F20:Week_1
- OpenWetWare. (2020). BIOL368/F20:Week 3. Retrieved September 22, 2020, from https://openwetware.org/wiki/BIOL368/F20:Week_3
- The Purdue Owl. (2018). Citation Chart. Retrieved September 15, 2020, https://owl.purdue.edu/owl/research_and_citation/using_research/documents/20180719CitationChart.pdf.
- Wan, Y., et al. (2020). Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. Journal of Virology, 54 (7), retrieved from https://doi.org/10.1128/JVI.00127-20.
- Pylaeva, S., Brehm, M., and Sebastiani, D. (2020). Salt Bridge in Aqueous Solution: Strong Structural Motifs but Weak Enthalpic Effect. Scientific Reports, 8 , retrieved from https://doi.org/10.1038/s41598-018-31935-z.
- Cammack, R., Atwood, T., Campbell, P., Howard, P., Smith, A., Vella, F., and Stirling, J. (2006). Oxford Dictionary of Biochemistry and Molecular Biology. Oxford University Press, 2 , Oxford, Retrieved from https://www.oxfordreference.com/view/10.1093/acref/9780198529170.001.0001/acref-9780198529170-e-14965?rskey=xODPBW&result=14881.
- Cammack, R., Atwood, T., Campbell, P., Howard, P., Smith, A., Vella, F., and Stirling, J. (2006). Oxford Dictionary of Biochemistry and Molecular Biology. Oxford University Press, 2 , Oxford, Retrieved from https://www.oxfordreference.com/view/10.1093/acref/9780198529170.001.0001/acref-9780198529170-e-17004?rskey=3vdqgd&result=16921.
- Cammack, R., Atwood, T., Campbell, P., Howard, P., Smith, A., Vella, F., and Stirling, J. (2006). Oxford Dictionary of Biochemistry and Molecular Biology. Oxford University Press, 2 , Oxford, Retrieved from https://www.oxfordreference.com/view/10.1093/acref/9780198529170.001.0001/acref-9780198529170-e-9938?rskey=AaY5WQ&result=9821.
- Biology Online. Dictionary: Residue, retrieved from https://www.biologyonline.com/dictionary/residue.
- Biology Online. Dictionary: ACE, retrieved from https://www.biologyonline.com/dictionary/ace.
- Dictionary.com. Dictionary: Palm Civet, retrieved from https://www.dictionary.com/browse/palm-civet.
- Merriam-Webster. Dictionary: MERS, retrieved from https://www.merriam-webster.com/dictionary/MERS.
- Classification of Protein Families. Spike Binding Domain, InterPro, retrieved from https://www.ebi.ac.uk/interpro/entry/InterPro/IPR036326/.
- Shiel, W. Jr. Medical Definition: Reservoir of Infection. Medicine Net, retrieved from https://www.medicinenet.com/script/main/art.asp?articlekey=14969.
"Except for what is noted above, this individual journal entry was completed by me and not copied from another source"Nathan R. Beshai (talk) 20:05, 22 September 2020 (PDT)