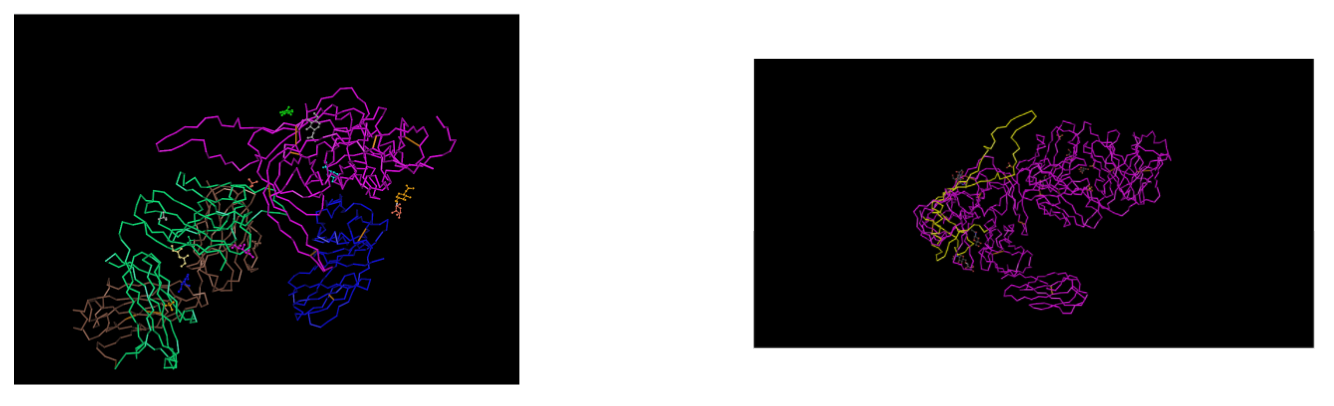Zachary T. Goldstein Week 10
From OpenWetWare
Jump to navigationJump to search
Electronic Notebook
Purpose
- The purpose of this assignment is to use programs learned in Week 9 to run a sequence analysis of the DNA and Amino Acid sequences of our newly identified subjects. We will use amino acid identifiers to see the types of changes and 3D protein modeling to see where the changes are located. All of this information will be documented and included in our Week 11 and Week 12 HIV Structure Presentations.
Methods/Results
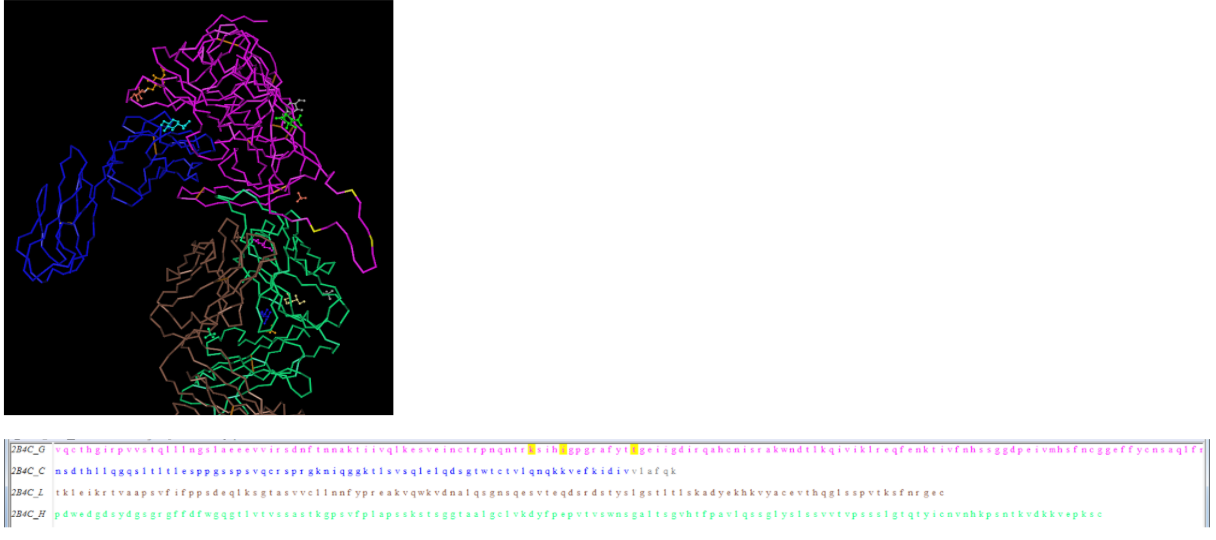
- Downloaded the 3D sequence of Huang et al. (2005) structure 2B4C gp120 protein using the Cn3D software site.
- Using amino acid sequences from the Markham data we identified the region that corresponds with the V3 region of the gp120 protein highlighted in yellow EVV-HSS region.
- We were able to identify the N and C terminals of the tertiary structures by selecting the amino acid and observing which portion of the protein got highlighted.
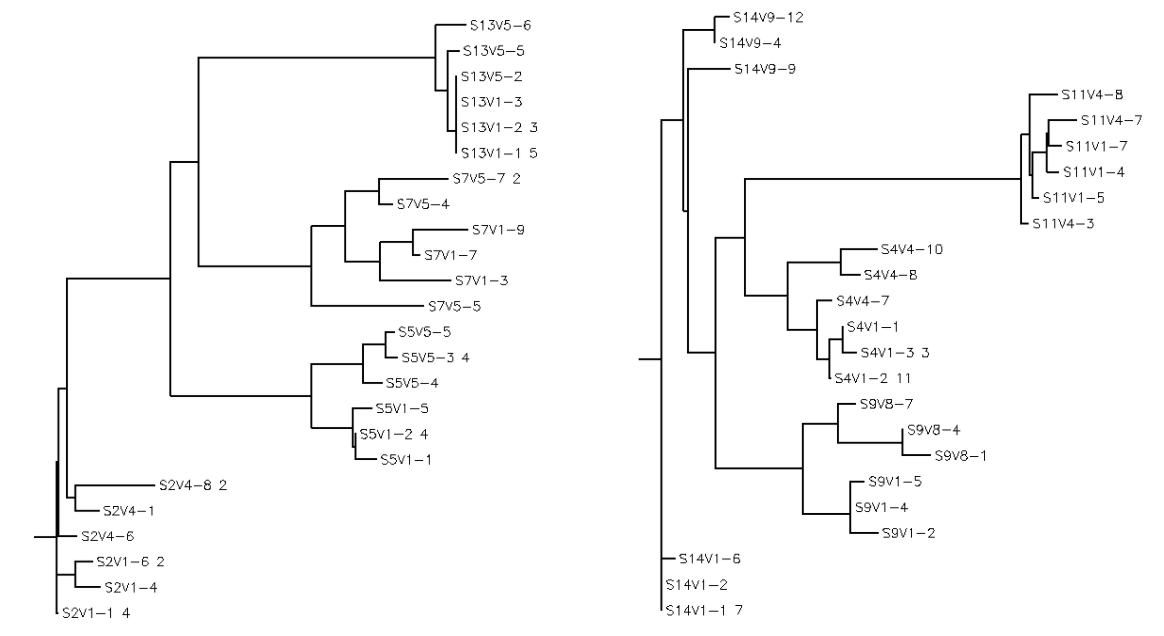
- Amino acid sequences from selected subjects were run under a multiple sequence alignment and a rooted tree was created.
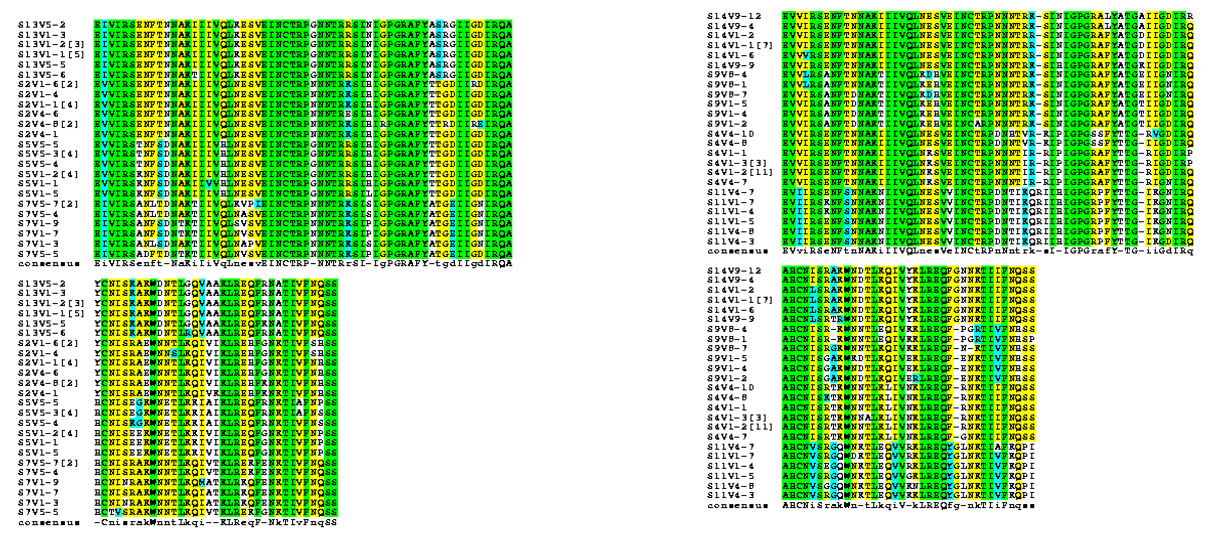
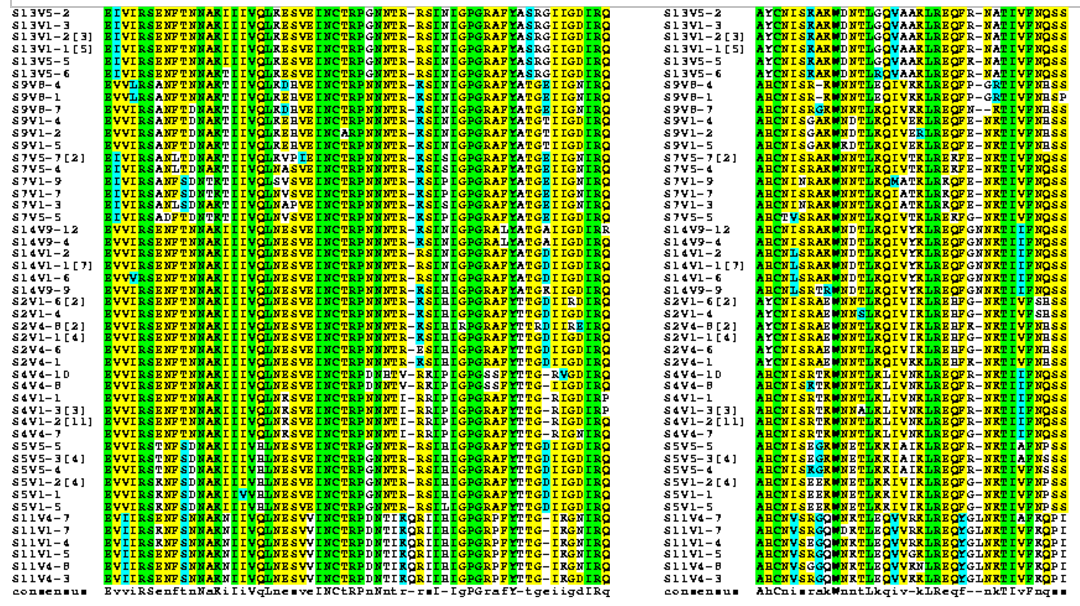
- Strong groups means like for like (acidic for acidic, etc...), weak groups means (hydrophobic for hydrophyllic), non-consensus means there is high variability between amino acids in each sequences. We decided to run two alignments to compare how many of these types of substitutions occur in the subjects with low dS/dN ratios vs. subjects with high dS/dN ratios.
- Alignment between clones of subjects 4, 9, 11, 14 (dS/dN=0) was run. We observed these amino acid sequences to have: Strong:20 Weak:6 Conserved:47 Non-Consensus:22
- Alignment between clones of subjects 2, 5, 7, 13 (dS/dN>0) was run. We observed these amino acid sequences to have: Strong:22 Weak:10 Conserved:51 Non-Consensus:12
- Alignments were rerun using the BoxShade tool to help us see where changes in amino acids were occurring along the sequences.
- Using information from the Journal Club articles, we selected 6 amino acids along the V3 region and observed their specific changes.
- We used the Protein Predictor website to understand how severe these changes in amino acids are.
- Problem: protein they crystallized is not the same as in Markham
- Turning strong weak conserved and nonconsensus data into ratios may be better for comparison. 10 more positions where there are changes in rapid progressor group. We have count and position data...are there different patterns where the nonconsenesus spots occur along the gene? We can use the 3D model to show where similarities and difference are in these positions.
- Positions along the protein where the sequence alignment is highly variable across all subjects therefore defined as "non-consensus": 37, 41, 50, 79, 86, 87.
- We selected these positions on the 3D model to highlight where they are positioned along the protein.
Data and Files
Conclusion
- Throughout this weeks assignment we established a better understanding of how amino acid changes along a protein effects its structure. Eventually I hope to gain a better understanding of how these structural changes actually alter protein function. Looking into the types of amino acids which are being substituted (ex: size, nonpolar, uncharged polar, acidic, basic, etc...) may lead to better predictions on the severity of these substitutions on protein function. Using the 3D crystalline structure of the gp160 protein we were able to project our "non-consensus" aka highly variable regions along the protein and compare these regions to "highly variable" regions defined in the Kwong and Andiranov articles from last class. We used the BoxShade alignment tool in biology workbench to highlight our non-consensus regions. So far, it looks as though there may be other variable regions along the gene that were not discussed in these papers, whose physical alterations (we think) may play a significant roll in HIV progression.
HIV Structure Project
- Link to slides saved Monday 11/7/16: HIV Structure Rough Draft
Acknowledgements
- I received help on this assignment from User: Jordan T. Detamore in class. I specifically received help with running amino acid alignments and analyzing our results using tools in Biology Workbench. Additionally over the weekend we worked outside of class on our GoogleDoc presentation for the week 11 presentations
- While I received help on this assignment everything on this page was completed by me and was not copied from anybody else.
Zachary T. Goldstein 17:42, 6 November 2016 (EST)Zachary T. Goldstein
References
- Week 10 Assignment
- NCBI Open Reading Frame Finder
- ExPASY Translate tool
- PredictProtein server
- NCBI Structure Database
- Huang et al. (2005) structure 2B4C
- Cn3D software site
All class assignments:
- Week 1 Assignment
- Week 2 Assignment
- Week 3 Assignment
- Week 4 Assignment
- Week 5 Assignment
- Week 6 Assignment
- Week 7 Assignment
- Week 8 Assignment
- Week 9 Assignment
- Week 10 Assignment
- Week 11 Assignment
- Week 14 Assignment
- Week 15 Assignment
All individual assignments:
- Zachary T. Goldstein Week 2
- Zachary T. Goldstein Week 3
- Zachary T. Goldstein Week 4
- Zachary T. Goldstein Week 5
- Zachary T. Goldstein Week 6
- Zachary T. Goldstein Week 7
- Zachary T. Goldstein Week 8
- Zachary T. Goldstein Week 9
- Zachary T. Goldstein Week 10
- Zachary T. Goldstein Week 11
- Zachary T. Goldstein Week 14
- Zachary T. Goldstein Week 15
All shared journals:
- BIOL368/F16:Class Journal Week 1
- BIOL368/F16:Class Journal Week 2
- BIOL368/F16:Class Journal Week 3
- BIOL368/F16:Class Journal Week 4
- BIOL368/F16:Class Journal Week 5
- BIOL368/F16:Class Journal Week 6
- BIOL368/F16:Class Journal Week 7
- BIOL368/F16:Class Journal Week 8
- BIOL368/F16:Class Journal Week 9
- BIOL368/F16:Class Journal Week 10
- BIOL368/F16:Class Journal Week 11
- BIOL368/F16:Class Journal Week 14
- BIOL368/F16:Class Journal Week 15