Todd:Chem3x11 ToddL11
Chem3x11 Lecture 11
This lecture is about elimination reactions.
(Back to the main teaching page)
Key concepts
- The two most common elimination mechanisms are E1 and E2.
- The mechanisms give rise to important issues of regio- and stereoselectivity
- Some E2 reactions are stereospecific, owing to very particular requirements of orbital overlap
Eliminations
An elimination reaction is where a small molecule leaves a carbon-containing molecule via the generation of a double bond.
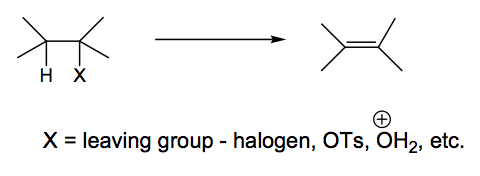
We will consider three mechanisms. They differ in the sequence of loss of the two components.
The E1 Reaction Mechanism
The X- leaves first, giving a carbocation, which then loses a proton to give the double bond. Rate limiting step is initial loss of anion. Hence rate only depends on concentration of starting material.
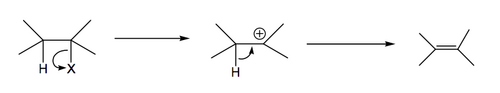
This mechanism is promoted when the intermediate carbocation is stable, e.g. when the carbocation is highly substituted, or in an allylic/benzylic position, or stabilised by an adjacent heteroatom. You've probably seen a very common example of E1 elimination where tertiary alcohols give alkenes in the presence of acid.

These substrates could also eliminate via an E2 mechanism (which we'll do in a minute) - it's just a question of selectivity. There is also usually a possibility of substitution reactions in many reactions. We just have to keep this in mind. As a rule of thumb we often see more elimination rather than substitution at higher temperatures.
Other groups are either less likely to react E1 or are unable to do so:

There is a regiochemical issue we ought to address. If there is a choice of double bonds being formed in the final step, we tend to see the more substituted double bond being formed. This arises from the more substituted transition state, containing the more substituted slightly charged carbon atoms of the double bond being formed. Here we see that the observed product is favoured kinetically and thermodynamically.
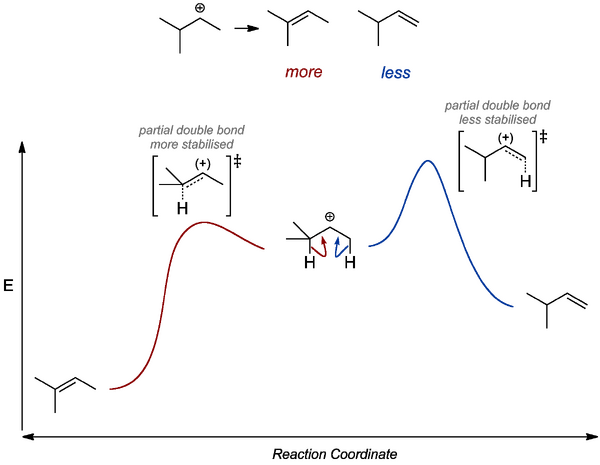
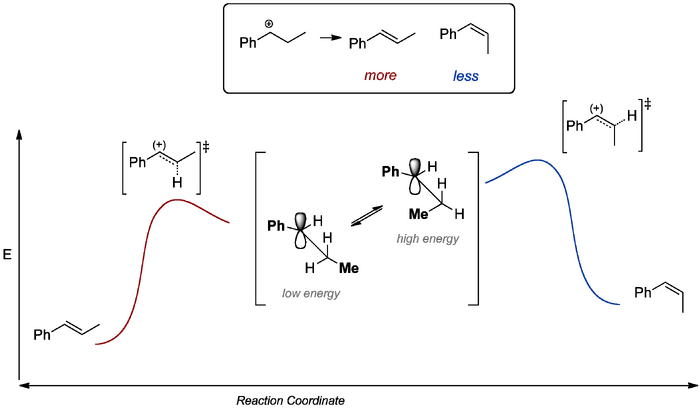
There is also a stereochemical issue we ought to address. E1 will tend to give E rather than Z products. Here we appeal to the difference in energies of the intermediate carbocations that must form (a steric argument) and extrapolate to the stabilities of the transition states going to the relevant products.
The E2 Reaction Mechanism
H+ and X- leave at the same time. We draw in a base as part of the reaction mechanism, which abstracts a proton. E2 processes will be favoured by strong bases (which are weak nucleophiles). We have seen this reaction in the elimination of hydrogen halides (dehydrohalogenation).
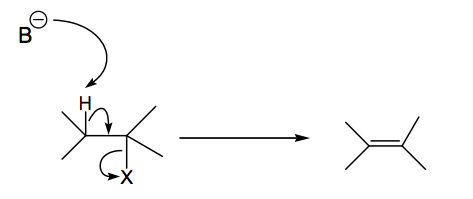
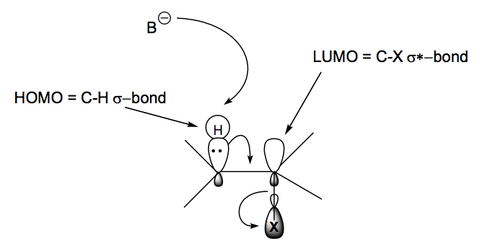
The one key thing about E2 reaction mechanisms is that the H and X groups must be antiperiplanar (APP) to eliminate. In acyclic systems that means a productive E2 reaction will only occur when the groups are in the right orientation. In cyclic systems in can mean some E2 reactions can't proceed if no appropriate APP arrangement is possible.
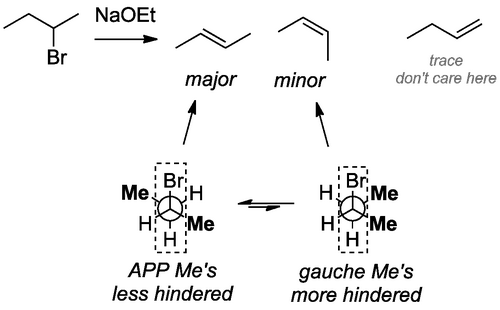
This neatly allows us to bring up the concepts of stereoselectivity and stereospecificity. Let's look at a stereoselective E2 elimination. In the case below there is a choice of which conformer of the starting material undergoes the E2 reaction. Both Newman projections show an APP arrangement of the groups involved in the elimination, but one is sterically less crowded than the other, so we obtain one product selectively. None are forbidden on orbital grounds.
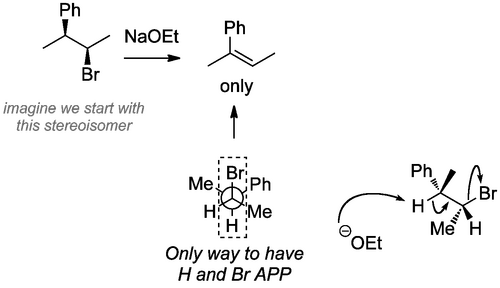
Now compare this reaction, where there is only one possible APP arrangement of the eliminating groups. Here we start with a single stereoisomer. Because there is only one possible APP arrangement of the eliminating groups, this reaction's outcome is absolutely controlled by the orbitals, so we say it's stereospecific. You need to be able to redraw the isomer such that the eliminating groups are APP, then eliminate and predict which stereoisomer of the double bond we obtain. Try this out using an isomer of the starting material here.
For cyclic systems, one may have to use a ring flip to orient groups in an APP arrangement suitable for E2 elimination. That may be possible, depending on what else is on the ring, of course.

Exceptions
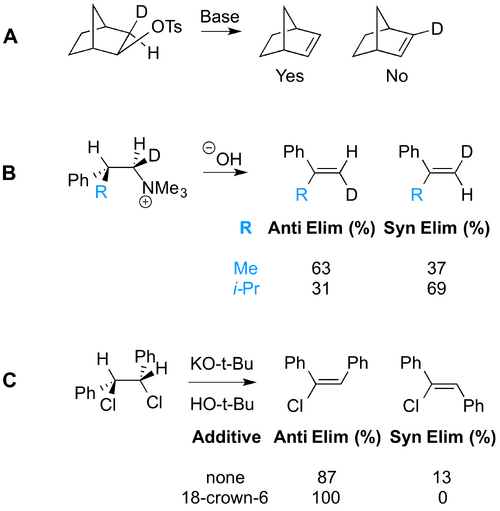
There are times when syn elimination becomes competitive. Below are three cases, described and explained in Anslyn and Dougherty (Chapter 10). See if you can see reasons why syn elimination might be occurring.
The E1cb Reaction Mechanism
Compared to E1 and E2 this mechanism is less important, but it has its moments. The idea is that H+ is abstracted by the base first (reversibly) to give a carbanion, which then loses X- - see the boxed part of the scheme below. Obviously this mechanism can happen when stabilised carbanions can form. There is one set of compounds where this happens a lot, and which is important in synthesis and biochemistry, namely β-hydroxy carbonyl compounds, which you've seen as the products of the aldol reaction.
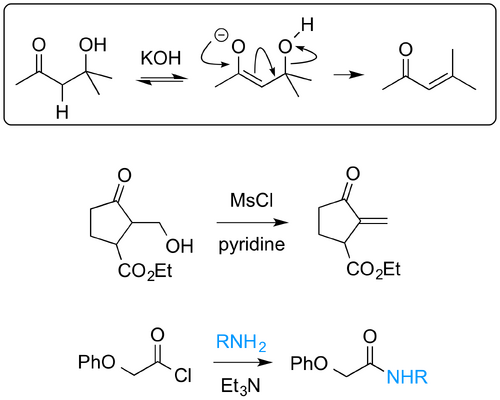
Clayden has a couple of nice examples, shown above, of E1cB in action (Chapter 17). See if you can work out the mechanism. The second example is harder than the first.
The Licence for This Page
Is CC-BY-3.0 meaning you can use whatever you want, provided you cite me.
