Todd:Chem3x11 Example Answer
An Example 3x11 Worked Answer
(Back to the main teaching page)
This question was on the 2011 paper:
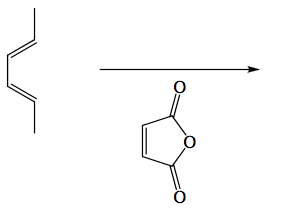
You are required to "Predict the product(s) formed ... and explain fully the reaction mechanism (where stereochemical issues are involved you will need to indicate the precise stereochemical outcome)" for 10 marks.
Remember in exam questions to state the obvious, before giving any complex explanations. Therefore the first thing to do is actually draw the 2D outcome.

Notice there there are stereochemical things we have to address here, but get the product connectivity and arrows established first. We have spent some time describing the orbital interactions behind pericyclic reactions, so let's deal with that.
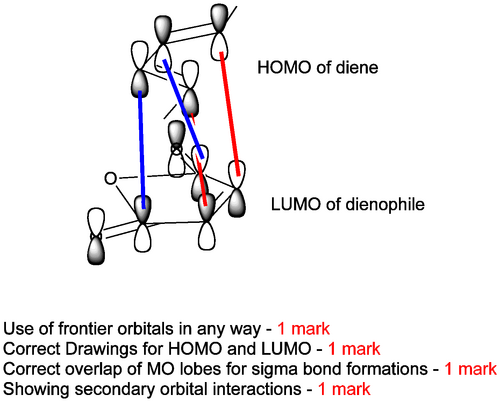
Once we've shown how the orbitals interact, we ought to explain the stereochemical outcome. We should show that the product formed is the endo product. We need to point out that the H's are cis in such a product. If we are careful in drawing our figure we can show that the methyls on the ends of the diene end up also cis, but anti to the H's - as shown. (The product is racemic - the stereochemistry shown is relative, not absolute). We didn't cover this fully in lectures, but it's another very useful feature of the DA reaction. You could say that though the endo product is formed, that is a kinetic outcome, and given the reversibility of everything we should eventually see the thermodynamic exo product if we push the reaction enough.

10 marks. Should take you 10 minutes.
