Biomod/2011/Harvard/HarvarDNAnos:Methods
<html> <head>
<style>
- column-one { display:none; width:0px;}
.container{background-color: #f5f5f5; margin-top:50px} .OWWNBcpCurrentDateFilled {display: none;}
- content {width: 0px; margin: 0 auto auto 0; padding: 1em 1em 1em 1em; align: center;}
- column-content {width: 0px; float: left; margin: 0 0 0 0;padding: 0;}
.firstHeading {display:none; width:0px;}
- globalWrapper{width:1280px; margin:auto}
body {background: #F0F0F0 !important;}
- column-one {display:none; width:0px;background-color: #f0f0f0;}
- content{border:none;margin: 0 0 0 0; padding: 1em 1em 1em 1em; position: center; width: 800px;background-color: #f0f0f0; }
.container{ width: 800px; margin: auto; background-color: #f0f0f0; text-align:justify; font-family: helvetica, arial, sans-serif; color:#f0f0f0; margin-top:25px; }
- bodyContent{ width: 1267px; align: center; background-color: #f0f0f0;}
- column-content{width: 1280px;background-color: #f0f0f0;}
.firstHeading { display:none;width:0px;background-color: #f0f0f0;}
- header{position: center; width: 800px;background-color: #f0f0f0;}
- footer{position: center; width:1280px;}
</style>
</head> </html>
<html>
</html>
Rectangular Box Methods
Go to Design...<html> </html> Go to Results...
Folding and Purifying Origami

Both the barrel and lid origami structures were prepared by mixing ~100 nM of the staples and ~20 nM of M13mp18 scaffold (NEB) with 5 mM Tris, 1 mM EDTA, and 15 mM magnesium chloride. Please see this protocol for more details. Here is a pipetting map that may help further clarify the mixing process and the determination of concentrations. The origami solution was then subjected to a thermal-annealing ramp, cooling from 80 to 64 degrees Celsius over 75 minutes and then further cooling from 64 to 24 degrees Celsius over 70 hours.
The resulting origami structures were prepared for imaging and further manipulation through a two-step process. First, the structures were separated from excess staples using a 1.5% agarose gel (with 10 mM magnesium chloride) run in 0.5x TBE 10mM magnesium chloride. The desired bands were manually excised and the origami structures were eluted from the gel using Squeeze 'n Freeze filters. After spinning the gel over the filter once, the gel was soaked in gel running buffer overnight before a final spin over the filter.
Next, the gel-purified solution was concentrated using a 100,000 kDa Amicon filter, whereby the solution was run through the filter at 14,000 g for 3.5 minutes, and then the filter was inverted and and spun down for 2.5 minutes at 1000 g, retrieving the captured and concentrated origami structures.
After concentration, analysis with the Nanodrop at the Nucleic Acid 260 nm absorbance reads ~0.2, which roughly translates into origami structures at a concentration of 2 nM.
See also: Folded Origami Results
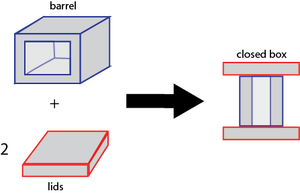
Closing the Box
 <html> </html>
<html> </html>
Part of why we like this design is that closing the box is very simple, elegant, and robust. To close the box, simply mix a two-fold excess of purified lids with purified boxes. Then, incubate at 35 degrees Celsius for 5 minutes and then cool to 24 degrees Celsius over 50 minutes. Alternatively, simply let sit at room temperature for 24 hours. The method is the same whether or not the barrels or lids have been loaded with gold.
See also: Closed Box Results
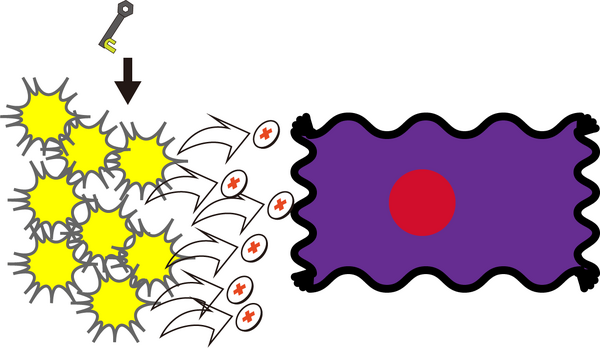
Opening the Box
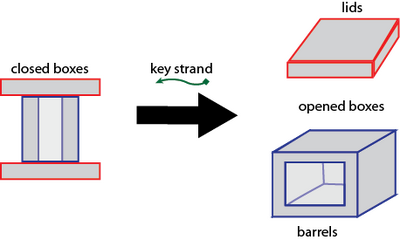
Opening the box proves quite straightforward as well. Simply introduce, to a closed box solution, the key strand at an approximately 500-fold excess to the concentration of lid lock strands. That is, the final concentration of key strands should equal 500 * concentration of closed boxes * 2 (lids/closed box) * 12 (locks strands/lid). Then, let sit at room temperature for 4 hours.
See also: Opened Box Results
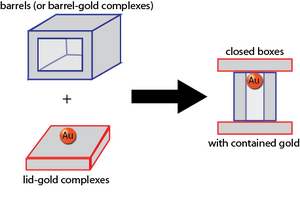
Loading Gold
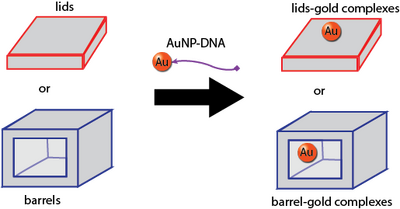
The protocol we use for conjugating 5-nm gold nanoparticles to ssDNA is secret. However, we will say that we used ssDNA with thiolated 3' ends, and you can refer to this alternative conjugation protocol. We used commercially available gold nanoparticles, although we also could have used our own Biomod/2011/Harvard/HarvarDNAnos:Results AuNP.
To purify away excess ssDNA after conjugation is complete, we washed 25 uL of the gold solution with water 5 times over a 50 kDa Amicon filter.
The protocols for subsequently attaching our AuNP-ssDNA conjugates to our lids and barrels is secret.
We purify excess, non-loaded AuNP-ssDNA from away from our origami after the attachment protocol and prior to imaging, but this purification protocol is also secret.
See also: Loading Results
Photocleavage
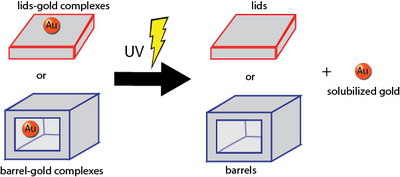
Photocleavage of photocleavable spacers for our photocleavage tests as well as for releasing gold from our lids was done according to these protocols.
See also: Releasing Cargo Results, Photocleavage Test Results
Future Steps
Sphere Methods
Go to Design...<html> </html> Go to Results...
Folding the Sphere
To fold our closed and open spheres, we followed the same method that Han et al. (2011) detailed in their supplemental information. More information about the folding of the sphere can be found below.
General Methods
AFM, TEM, and Gels
The protocols we used to collect data and images are all on our protocols page.