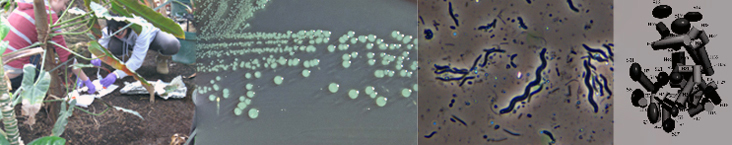
BISC209/S13: Motility
Techniques to examine Motility
Flagella are bacterial structures that allow directed movement, called motility. Motility enables bacteria to move towards favorable environments and away from unfavorable ones and is sometimes important in the characterization and identification of bacteria. Arrangement of flagella varies among species. A flagellum may occur singly at one end, or there may be more than one flagella at one or both ends (polar). Flagella may occur in tufts, or they can be arranged all around the cell (peritrichous). Not all motile bacteria have flagella and many bacteria are non-motile. There are different ways to examine motility or motility organelles. You should begin with the motility part of a soft agar deep test and if that is positive, do a wet mount to confirm motility and/or do a flagella stain to see if you can see flagella.
Soft Agar Deep Test for motility
This special media relies on the ability of motile bacteria to move through a tube of semisolid medium. The growth of motile bacteria in such a tube will produce turbidity throughout the solid medium, whereas non-motile organisms will grow only along the line of inoculation. The media we will use has 0.35% agar instead of the usual 1.5%
PROTOCOL:
Inoculating the a soft agar deep tube involves a technique you have not yet practiced. It is very similar to inoculations using the flame sterilized loop, but you will use an inoculating needle (the wire extending from the handle will not have a loop on the end). After picking up a visible amount of your isolate on the tip of a flame sterilized inoculating needle, you will stab it deeply into the center of the medium in the tube, stopping just before the bottom of the tube or, if you are running out of needle, stab it until the you are almost to the end of the needle.
Withdraw the needle through the same inoculation channel. This procedure is also known as "making a soft agar deep".
Inoculate a positive control organism using the same technique.
Incubate for 24-72 hours (or as appropriate)
Motile organisms (such as E. coli) will exhibit growth radiating from the stab inoculation line.
Non motile organisms (such as Staphylococci or Streptococci) will exhibit growth only along the stab inoculation line.
Interpretation:
Motility detection is possible due to the semisolid nature (low concentration of agar) of "soft" agar. Growth radiating out from the central stab inoculation line indicates that the test organism is motile. The motility test should be assessed first. Motile organisms will exhibit growth radiating from the stab inoculation line. Non motile organisms will exhibit growth only along the stab inoculation line.
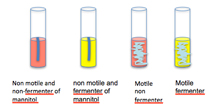
Interpreting the ability to ferment mannitol from mannitol nitrate motility soft agar medium (MNM):
The indicator phenol red will show a pH change indicated by a color change from red to yellow (throughout the tube) allowing the interpretation that the organism is able to ferment mannitol as a carbon source.
Interpreting the ability to reduce nitrate to nitrite:
Add a few drops of Gries reagents (follow manufacturer's directions) to cultures of bacteria grown in MNM medium. If the liquid at the surface turns red (a darker red than the medium, if it is red), that is a positive test for nitrate reduction to nitrite. No color change is a negative reaction.
Hanging Drop and motility
The direct observation of bacterial motility is possible using the hanging drop technique. Flagellated organisms can move across the field of view at 10 body lengths per second or faster. Other forms of motility such as twitching or gliding require more patience to observe. The small size of bacteria under 400x magnification make this technique somewhat tricky but the satisfaction of seeing living organisms is worth the trouble. If you suspect your organism has flagella, try this technique.
Procedure:
- Use a toothpick to apply a tiny drop of vaseline to the 4 corners of a coverslip
- Aseptically transfer 1-2 loops of a log phase broth of the organism to the center of the coverslip.
- Turn a clean DEPRESSION slide so that the depressions are facing down and carefully place one of the depressions over the drop containing your organism. The vaseline on the corners of the coverslip should adhere to the slide, allowing you to flip the slide over with the coverslip attached. You can now view your organism on the Microscope using ONLY 10 and then 40x objectives. Start with the 10x objective, cut the light to a minimum, and try to find the edge of the drop. Ask your instructor to check that you are seeing the drop and not scratches in the slide or coverslip. Place the edge of the drop in the center of your field of view and move to the 40x objective. Do not add oil to view the hanging drop or try to use the 100x objective, you might break or scratch the 100x objective. You should be able to see TINY bodies either vibrating in place or making progress across the field of view. The organism is motile if you see it actually moving from place to place across your field of view.
Flagella stain
Flagella are fragile protein structures that are too thin to be resolved by the human eye using a brightfield microscope. However, if coated with stain and mordant, the diameter increases, sometimes enough to exceed the resolution limits of the human eye. Because motility is important functionally, it is worthwhile to determine if your bacterial isolates possess flagella. Since the flagella are quite fragile and often fall off the cell during the culture or staining process (or they may not take on enough stain complex to be thick enough to view) a negative flagella stain is not conclusive of non-motility. Use this stain in conjunction with other functional tests for motility such as the hanging drop and/or SIM test.
The advantage to staining the bacterial flagella, rather than simply relying on the functional tests for motility, lies in the stain's ability to reveal number and arrangement of flagella. It is common for a motile species to have more than one per cell. Flagella arrangement is quite variable among bacterial species. The most common arrangements you might observe are peritrichous (many flagella positioned all around the bacterial cell) and polar (one or more found at one or both ends of a cell). There are flagellated bacteria in many genera including: Aeromonas, Proteus, Bacillus and others.
FLAGELLA STAIN Procedure:
(adapted from: Murray, R. G. E., R. N. Doetsch, and C. F. Robinow. 1994. Light microscopy. In P. Gephardt, R. G. E. Murray, W. A.Wood, and N. R. Krieg (ed.), Methods for general molecular bacteriology. American Society for Microbiology, Washington, DC. and MelliesReed, Jay. Bacterial Flagella Stain Protocol Resource Type: ASM Curriculum: Protocol Publication Date: 9/8/2008)
1. Clean a new slide with BonAmi cleanser to remove any film or oils that might be on the slide. You will need 1 cleaned slide for each bacterial isolate you wish to examine. Dry it and run it through the flame of your Bunsen burner to remove any oils from your fingers. Cool with out touching the slide.
2. Using a flame sterilized loop, transfer a small amount of growth from an agar plate or slant cultures into 2ml water in test tube (does not need to be sterile). Mix gently and check that the suspension is only slightly cloudy. Using too much inoculum will cause make it difficult to visualize the flagella. Add more water if you need to dilute the innoculum.
3. Pipet 5 μL of the culture suspension to one end of the slide using your P20 micropipet .
4. Spread the suspension gently by holding the pipet sideways over the slide and rolling the pipet tip gently over the liquid once.
5. Discard the pipet tip in the autoclave bag.
6. Air dry thoroughly. Do not heat fix.
Presque Isle Cultures flagella stain
7. Flood the smear area of the slide with Presque Isle Cultures Solution I, the mordant. Incubate at room temperature for 4 minutes.
8. Gently rinse with distilled water. Shake excess water from slide.
9. Flood with Presque Isle Cultures Solution II, the silver stain.
10.Use your clothes pin slide holder to pass the slide over, rather than in, a Bunsen burner flame by moving slide back and forth slowly, just until steam is emitted. Be careful not to overheat sample, as excess heat will destroy the flagella. Incubate at room temperature for 4 minutes.
11.Rinse in a gentle stream of distilled water. Carefully blot dry with bibulous paper or air dry.
12.View using oil immersion, at 1,000x magnification, by brightfield microscopy. Bacteria and flagella will appear golden brown. If too much stain was applied and the microscope slide was not really clean, it is difficult to find the cells and flagella.
The ingredients in the stain and mordant are proprietary reagents of Presque Isle Cultures, P.O. Box 8191, Erie, PA 16505; http://www.picultures.com