Summary
Our mission is to bridge the gap between clinical needs and engineering advances by developing multimodal imaging systems and multifunctional theranostic agents for intraoperative imaging and image-guided therapy.
Multimodal imaging (including dual-mode imaging) represents the seamless integration of multiple imaging modalities into a single unit for simultaneous detection of various tissue parameters. Traditional biomedical imaging tools use static single modality to target limited disease markers. Each modality contributes to only a small piece of the complex puzzle. Recently, hybrid imaging systems, such as positron emission tomography-computed tomography (PET-CT), single photon emission computed tomography (SPECT)-CT, magnetic resonance imaging (MRI)-PET, MRI-near infrared (NIR), and NIR-ultrasound(US), are explored for simultaneous detection of tissue structural, functional, and molecular characteristics. However, broader clinical applications of these hybrid systems are challenged by multiple limitations, such as the significantly added cost and complexity, the compatibility and co-registration issues between different modalities, and the measurement biases/artifacts due to patient-to-patient variations, tissue heterogeneities, and test condition changes. To overcome these limitations, we proposed a multimodal dynamic schema for real-time detection of tissue dynamic characteristics in response to external stimuli. Our current technical focus is to integrate optical and US imaging modalities. However, the technology developed in my lab can be extended to integrate many other modalities such as PET, thermographic imaging, and photoacoustic tomography.
Multifunctional theranostics refers to multimodal approaches to deliver combined diagnostic and therapeutic agents to the targeted sites with high specificity and in adequate concentrations for combined diagnosis and treatment of diseases in a single sitting. We are developing multifunctional theranostic agents with high disease-targeting specificity and strong imaging contrasts in multiple image modalities. To facilitate the future translation of these theranostic agents from the benchtop to the bedside, we have considered clinical safety and efficacy at the very beginning. Materials used to construct these agents are either FDA approved biodegradable and biocompatible materials or materials without reported toxicity.
Image-guided therapy refers to the acquisition and manipulation of biomedical images to guide medical interventions. Clinical safety and efficiency of image-guided therapy heavily rely on the accurate detection of tissue anomalies and the effective protection of the surrounding normal tissue structures. Currently available image-guided therapeutic systems are typically large, expensive, and incompatible with existing clinical procedures. We aim at developing handheld imaging systems to guide the intervention and monitor the response in the operating room.
We are currently working on seven projects: (1)Ultrasound and near infrared dual-mode dynamic imaging of breast cancer, (2)Multifunctional contrast agents for cancer targeting and multimodal imaging, (3)Microbubble-assisted synergistic epigenetic therapy, (4)Image-guided cancer surgery, (5)Ablation margin assessment in cancer, (6)Dual-mode imaging of cutaneous tissue oxygenation and vascular reactivity, and (7)Intravitreous injection of drug-loaded microbubbles for anti-VEGF therapy. These projects are developed in close collaboration with physicians and clinical researchers at OSU Medical Center with careful considerations of clinical impact, technical feasibility, and scientific merit. These projects are interconnected with each other to form an integrated platform for multimodal imaging and image-guided therapy.
Ultrasound and near infrared dual-mode dynamic imaging of breast cancer
Objective: To develop and validate handheld dynamic imaging techniques for non-invasive characterization of optical, physiological and physical properties of suspicious breast lesions.
Hypothesis: Breast tumors can be characterized based on their structural and functional dynamic characteristics in response to external compressive stimuli.
Clinical problem: Early detection and appropriate treatment of breast cancer is associated with the improved long-term survival. However, existing breast imaging tools such as mammography do not provide functional information relevant to tumor development and progression. NIR diffuse optical tomography (DOT) has been previously investigated for noninvasive functional imaging of breast cancer. However, the accuracy for NIR measurement is vulnerable to test condition changes, tissue heterogeneities, and patient to patient variations.
Methods and results: We propose to characterize breast lesions based on their structural and functional dynamics in response to external compressive stimuli. We have developed a handheld probe with embedded pressure sensors and optoelectronic components for noninvasive monitoring of tissue oxygen, hemoglobin concentration and their dynamic changes in response to external compression (Fig 1a). We have also tested the compression-induced tumor structural and functional dynamics on a cancer xenograft model (Fig 1b). Motivated by the preclinical results, we have further developed a handheld probe integrating US and NIR modalities for simultaneous characterization of tumor structural and functional dynamics in response to mechanical compression (Fig 1c-1d). The dynamic breast imaging technique has been tested in a 50-subject clinical trial at the OSU James Breast Health Center (IRB protocol #2005C0005). Another clinical trial has also been carried out to validate the US and NIR dual-mode probe (Fig 1e, IRB protocol #2008C0006).
Research support: Wallace Coulter Foundation, OSU BME, OSU Surgery.

Fig 1. (a) A handheld probe for dynamic near infrared imaging. (b) Benchtop setup for the study of compression-induced tumor tissue dynamics in a cancer xenograft model. (c) CAD model of a NIR-US dual-mode probe. (d) Benchtop validation of the NIR/US dual-mode probe. (e) Clinical setup for dual-mode dynamic breast imaging.
Multifunctional contrast agents for cancer targeting and multimodal imaging
Objective: To synthesize biodegradable contrast agents for cancer-specific multimodal imaging.
Hypothesis: Multifunctional microbubbles, nanobubbles, and fluorescence agents conjugated with cancer-specific antibodies will enable effective cancer targeting and imaging.
Clinical problem: Simultaneous assessment of tissue structural, functional and molecular characteristics is important for accurate detection of tumor boundaries and occult metastatic diseases. However, many existing cancer imaging tools use a single modality to reveal a small piece in the complex puzzle of tumor development and progression. The ultimate need to bridge the gaps in cancer detection and imaging remains a significant challenge for oncologists, and once overcome, may ultimately revolutionize the cancer-specific imaging technologies.
Methods and results: We have conjugated CC49 antibody with different imaging markers and tested various cancer-targeting strategies in LS174T human colorectal cancer cell cultures and xenograft mice. CC49 targets TAG-72, a human glycoprotein complex over-expressed in the extracellular space of many epithelial cancers, including colorectal, pancreatic, breast, and ovarian cancers (Fig 2a). We have radiolabelled CC49 with 124I for PET-CT imaging of the colorectal cancer xenograft (Fig 2b). We have conjugated CC49 antibody with Cy7, an Indocyanine Green (ICG) derivative, for in vivo fluorescence imaging (Fig 2c). We have also synthesized multimodal microbubbles (size: ~3um) and nanobubbles (size: ~300nm) by encapsulating various imaging agents, such as ICG, India ink, and TexasRed, in a poly-lactic-co-glycolic acid (PLGA) shell using a modified double emulsion method. TexasRed encapsulated nanobubbles have been conjugated with CC49 for cancer targeting and imaging. Fluorescence images in Figs 2f-2g show that CC49 conjugated nanobubbles effectively bind with cancer cells while nanobubble without conjugation do not bind well with cancer cells. Encapsulating multiple imaging agents in PLGA microbubbles and nanobubbles also enables quantitative multimodal imaging. As an example, we encapsulated ICG in microbubbles for simultaneous US and fluorescence imaging (Figs a and b). Although ICG is a FDA approved contrast agent, its clinical application is limited by the inconsistent spectral characteristics due to aggregation and molecular interaction in blood stream. By encapsulating ICG in PLGA microbubbles, we have demonstrated consistent fluorescence spectra in water and in plasma (Fig 3c). We have also encapsulated India ink in PLGA micro bubbles and nanobubbles for simultaneous US and photoacoustic imaging (Figs 3d-3g).
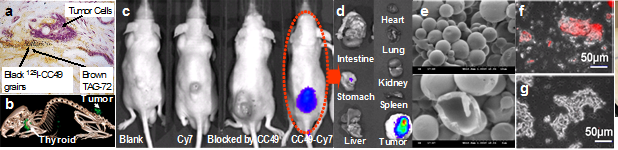
Fig 2. (a) Histological image shows extracellular expression of TAG-72. (b) PET-CT image of cancer xenograft after i.v. injection of 124I labeled CC49. (c) Fluorescence images of colorectal cancer xenograft mice at 96 hr postinjection of the following agents: Blank (PBS control), Cy7, Blocked by CC49 (excessive CC49 followed by CC49-Cy7), and CC49-Cy7. (d) Fluorescence images of dissected organs at 96 hr post-injection of CC49-Cy7. (e) Scanning electron microscopic (SEM) images of PLGA microbubbles. (f-g) Fluorescence microscopic images of colorectal cancer cells show that: (f) CC49 conjugated nanobubbles bind with cancer cells very well, (g) nanobubbles without CC49 conjugation do not bind with cancer cells.
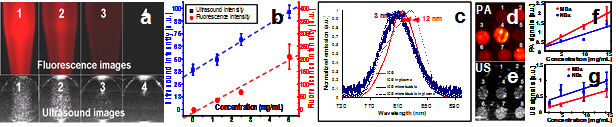
Fig 3. (a) Simultaneous fluorescence and US images of centrifuge tubes filled with ICG-loaded microbubbles at different concentrations. (b) US and fluorescence intensities obtained from Fig a are linearly correlated with the microbubble concentration. (c) Plasma and water suspensions of ICG-loaded microbubbles show consistent the emission spectra (blue lines), while ICG solution in plasma has significant distorted spectrum (red lines). (d-e) photo-acoustic and US images of tumor simulators made of microbubbles and nanobubbles at different concentrations. (f) Photoacoustic and US intensities are linearly correlated with microbubble and nanobubble concentrations.
Microbubble-assisted synergistic epigenetic therapy
Objective: To synthesize multifunctional drug-loaded microbubbles for image-guided synergistic epigenetic therapies in cancer.
Hypothesis: Microbubble-assisted epigenetic therapy in combination with PI3K/AKT signaling inhibition will significantly enhance the anti-cancer synergy.
Clinical problem: Recent research in epigenetic alterations offers a new opportunity for cancer detection, prognosis, and treatment. Our collaborator has demonstrated the synergistic anti-cancer effect for the combinatory therapy of DNA methylation inhibition, histone deacetylase inhibition, and AKT signaling inhibition in many breast cancer cell lines. However, clinical translation of this promising therapy in treating solid tumors is hindered by the lack of an effective drug delivery method. In this regard, the efficiency of the in vivo combinatory therapy is limited by the different release profiles for individual drug components.
Methods and results: We propose to load combinatory epigenetic drugs in PLGA microbubbles for targeted delivery and controlled release of synergistic therapies. We also propose to conjugate anti-VEGFR2 antibody with microbubbles to target tumor vasculature and encapsulate imaging agents in microbubbles for image-guided drug delivery. To demonstrate the technical feasibility, we have successfully encapsulated 5-aza-2’-deoxycytidine (DAC, a DNA demethylation reagent), trichostatin A (TSA, a histone deacetylase inhibitor), and LY294002 (PI3Ki, a PI3K/AKT inhibitor) in PLGA micro-particulates (Fig 4a) and intratumorally injected into a sample tumor of a T-47D xenograft mouse. In comparison, the same dose of PLGA microparticulates without drugs was intratumorally injected into the control tumor of the same type. The sample and the control tumors were dissected two week post-injection for pathological analysis. According to H&E staining (Fig 4b) and Ki67 staining (Fig 4d), the sample tumor (i.e., injected with drug-loaded PLGA microparticulates) does not show cancer proliferation or invasive cancer cells beyond the tumor boundary. In comparison, the control tumor (i.e., injected with PLGA microparticulates only) shows cancer proliferation and the invasive growth of cancer cells beyond the tumor boundary (Figs 4c and 4e).
Support: National Cancer Institute.
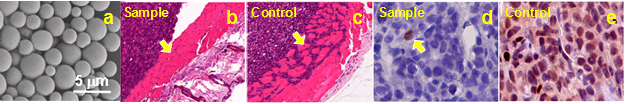
Fig 4. (a) A representative Scanning Electron Microscope (SEM) image of DAC-loaded microbubbles. H&E staining show the constrained cancer cells within the tumor boundary for the sample tumor (b), and the invasive cancer cells beyond the tumor boundary (c). Yellow arrows are tumor boundaries. Ki67 staining show only one proliferated cancer cell in the sample tumor (d, yellow arrow), but a large amount of proliferated cancer cells in the control tumor (e).
Image-guided cancer surgery
Ablation margin assessment in cancer
Dual-mode imaging of cutaneous tissue oxygenation and vascular reactivity
Intravitreous injection of drug-loaded microbubbles for anti-VEGF therapy
|