User:Mbennie/Notebook/Lab Notebook/Notebook/2007/08/13
From OpenWetWare
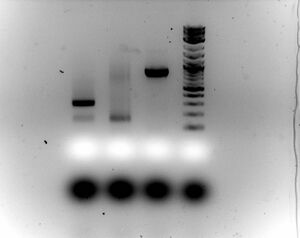
- Gel
- Ran 1.5% gel for 30 minutes at 100V with samples (5ul sample with 2ul of loading dye)
- Something is going wrong between first ligation and second ligation
- Digest
- Template: 3ul DNA, 2ul NEB2, .2ul BSA, .5ul of EcoRI and Pst1, rest water (20ul)
- IgAbc F (B + C with D)
- IgAbc R (C + D with B)
- PCR didn't work, threw away
- Template: 3ul DNA, 2ul NEB2, .2ul BSA, .5ul of EcoRI and Pst1, rest water (20ul)
- Antibody Tag Primers
- Ordered (more info later)
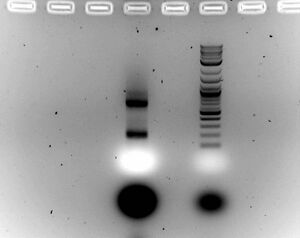
- Gel
- Ran 1.5% gel for 30 minutes at 100V with samples (20ul sample with 5ul of loading dye)
- Cut band at about 700bp of B + C for gel purification
- Gel Purification
- QIAEX gel purification
- Eluted in 20ul of water
- Spec
- B + C: 82.5 ng/ul
- PCR
- Template: 40ul PCR Supermix, .4ul of each primer, 1ul gel purification product
- B + C: Run with normal primers (IgAb-F and Mut_Pst1b-R)
- B + C diagnostic: Run with normal primers plus Mut_Pst1a-R
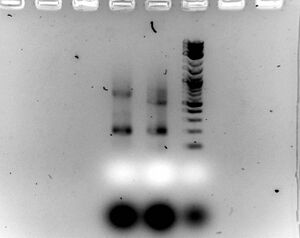
- Gel
- Ran 1.5% gel for 30 minutes at 100V with samples (5ul sample with 2ul of loading dye)
- Looks like the diagnostic PCR has a band 100bp shorter than B+C
- Looks promising
- What is creating this band at 200bp?
- Digest
- Template (20ul rxns): 3ul DNA (of each), 2ul NEB4, .5ul Sap1, rest water
- B + C with D
- Thermocycler protocol: 1hr@37C, 20mins@65C
- Template (20ul rxns): 3ul DNA (of each), 2ul NEB4, .5ul Sap1, rest water
- Ligation
- Performed on digest tubes above
- Template (50ul rxns): 20ul digest, 5ul ligase buffer, .5ul ligase, rest water
- Thermocycler protocol: 30mins@roomtemp,10mins@65C