DropBase:droplet electrosorting 5

Overview
Description
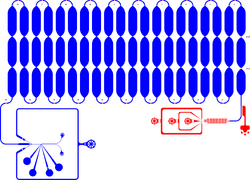
Integrated microfluidic chip device for droplet generation, incubation and fluorescence-activated droplet sorting (FADS) of ≈ 11 pL-sized droplets (in-line incubation). Incubation time in the delay line is ≈ 20–30 min. This long design is suitable to measure the reaction progress in droplets to estimate the required delay line length for stringent droplet sorting. Shallow contrictions in the delay line promote droplet mixing and allow monitoring of droplet fluorescence at different time points of incubation. Red: 15 µm depth. Blue: 46–48 µm depth.
Reference
D. Schnettler Fernández, O. J. Klein, T. S. Kaminski, P.-Y. Colin, F. Hollfelder. Ultrahigh-throughput directed evolution of a metal-free α/β-hydrolase with a Cys-His-Asp triad into an efficient phosphotriesterase. unpublished
Submitted by: David Schnettler Fernández
Downloads
Usage Notes
Please enter any comments that other users may find useful below this note (such as flow rates that worked well for particular oil/aqueous phases). When providing usage notes please provide as much detail as useful. We would request that you 'sign' any comments with your initials.
Optics and electronics of the microfluidic on-chip sorting device were set-up as previously described ([1], [2]). Monodisperse water-in-oil microdroplets were generated in the flow-focusing module of the chip. The device was connected via PE tubing (0.38 mm inner diameter, 1.09 mm outer diameter; Adtech Polymer Engineering) to glass syringes (100 μL and 1 mL; SGE Analytics), which were driven by syringe pumps (neMESYS, Cetoni, Germany). Fluorocarbon oil (3M Novec HFE-7500) containing 0.5 % (w/w) surfactant (008-FluoroSurfactant; RAN Biotechnologies, USA) served as oil phase. The two aqueous streams were supplied with the cell solution and with a substrate solution containing lysis agents (0.7× BugBuster protein extraction reagent, Merck Millipore; 60 kU/mL rLysozyme, Novagen) in droplet assay buffer, respectively. Droplet formation was monitored using an inverted microscope (SP98I, Brunel Microscopes, UK) with a high-speed camera (Phantom Miro eX4, Vision Research, USA). The generated emulsions were channelled through the delay line for incubation at room temperature for a precisely defined time (here: ≈ 5 min, depending on the chosen flow rates). For tight spacing of the droplets, required for even mixing and homogenous incubation times, oil was removed through an oil extractor. At the end of the delay line, droplets were injected into the sorting module. To enable precise sorting of single droplets, the distance between the droplets was increased by injection of spacing oil (Novec HFE-7500, 3M, USA) into the device. The asymmetric Y- shaped junction in the device ensures that all droplets automatically flow into the waste channel, unless deviated by an electrical pulse into the sorting channel. A 488-nm laser was focused 100 μm upstream of the sorting junction through a 40× microscope objective (UPlanFLN, Olympus, Japan) for fluorophore excitation and the emitted fluorescent light was collected and amplified using photomultiplier tubes (PMM02, Thorlabs, USA). Whenever the fluorescence peak reached a user-defined threshold, an electric field was applied by the two electrodes on the sorting device, attracting the highly fluorescent droplet towards the narrower sorting channel. Droplets were sorted into a collection tube pre-filled with 100 μL nuclease-free water. On this chip, flow rates were 7.5 µL/h for the aqueous phases, 25 µL/h for the oil phase, ≈ -10 µL/h for the oil extractor, and ≈ 300 µL/h for the spacing oil, resulting in a droplet volume of ≈ 11 pL. Note: During device operation, increase and adjust flow rates very slowly and allow sufficient time for pressure equilibration to avoid building up back-pressure in the delay line and thus ensure precise, even flow rates. DSF