Biomod/2013/LMU/nanodiamonds
<html>
<head>
<link href='http://fonts.googleapis.com/css?family=Open+Sans' rel='stylesheet' type='text/css'>
</head>
<style>
/*BUT ACTUALLY*/ body {
font-family: 'Open Sans', sans-serif; overflow-y: scroll;
}
.container {
background-color: #0000ff; margin-top:10px
} .OWWNBcpCurrentDateFilled { display: none; }
h5 {
font-family: 'Open Sans', sans-serif; font-size: 12px; font-style: normal; text-align: center; margin:0px; padding:0px;
}
- column-content
{
width: 5px; float: left; margin: 0 0 0 0; padding: 0;
} .firstHeading {
display:none; width:0px;
}
- column-one
{
display:none; width:0px; background-color: #ffffff;
}
- globalWrapper
{
width: 1000px; background-color: #ffffff; margin-left: auto; margin-right: auto
}
- content
{
margin: 0 0 0 0; align: center; padding: 12px 12px 12px 12px; width: 976px; background-color: #ffffff; border: 0;
}
- bodyContent
{
width: 950px; align: center; background-color: #ffffff;
}
- column-content
{
width: 1000px; background-color: #ffffff;
}
- footer
{
position: center; width: 1000px;
} @media screen {
body { background: #000000 0 0 no-repeat; /* changed default background */ }
}
- menu
{
position: fixed; float: left; width: 190px; padding: 5px; background-color: #FFFFFF; font-size: 15px; font-style: normal; text-align: left; margin:0px; padding:0px;
}
- pagecontent
{
float: right; width: 720px; margin-left: 300px; min-height: 500px
}
- toc { display: none; }
/*Expanding list*/ ul { list-style: disc; } ul.a { list-style: none; }
- exp li ul { display: none; }
- exp li:hover ul { display: block; }
- exp li a:active ul { display: block; }
a:link {color:#275ca8;} a:visited {color:#54c3f1;} /* visited link */ a:hover {color:#c2e5f8;} /* mouse over link */ a:active {color:#214897; } /* selected link */
</style> <body> </body> </html>
Properties of Nanodiamonds

Nanodiamonds are allotropes (sp3) of carbon and possess a series of distinct properties compared to other carbon–based materials. Their outstanding characteristics include extreme hardness, high thermal conductivity, transparency at all optical wavelengths, biocompatibility, and chemical robustness [1]. Pure diamond is an electric insulator while doped diamond is a semiconductor with a large bandgap (5.5 eV at room temperature). In addition doped impurities can create optically active defects – also called color centers – within the diamond. A nitrogen vacancy center (NV) consists of two adjacent positions in the atomic lattice of a diamond one replaced by a nitrogen atom while the other remains vacant. Under excitation with an appropriate light source, NV centers are a reliable single-photon source. The emitted photons carry defined spin information related to the spin state of the NV center. During the production of nanodiamonds by detonation of TNT-hexogen mixtures nitrogen defects are added. [2].
Although nanodiamonds are sp3 allotropes of carbon materials many residual groups, such as hydroxyl, carboxyl, lactone, ketone, exist on nanodiamonds due to their high surface energy. These groups provide initial functional sites for further modification.
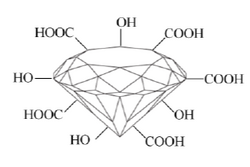
The surface modification of the nanodiamonds can be divided into two main categories: wet methods [3, 4] and dry methods [5, 6].The dry methods usually employ gas plasma or high temperatures to remove the surface impurities and embedded functional radicals. The wet methods usually employ mineral acids to oxidize the surface and then further conjugate the oxidized surfaces with other groups by non-covalent or covalent interaction.
Both procedures modify the nanodiamonds surface by introducing carboxyl or amino groups to enable an attachment. For our project, we used nanodiamonds that were already modified by wet method by Tanja Weil's group from Ulm Univeristy.
References
-
Krueger, A. "The structure and reactivity of nanoscale diamond", J. Mater. Chem., 2008, 18, 1485-1492.
-
Osawa, E. "Monodisperse single nanodiamond particulates", Pure Appl. Chem., 2008, 80, 1365–1379.
-
Ushizawa, K. et. al. "Covalent immobilization of DNA on diamond and its verification by diffuse reflectance infrared spectroscopy", Chem. Phys. Lett., 2002, 351, 105-108
-
Kruger, A. et. al., "Surface functionalisation of detonation diamond suitable for biological applications", J. Mater. Chem., 2006, 16, 2322-2328.
-
Wenmackers, S. et. al., "Diamond-based DNA sensors: surface functionalization and read-out strategies", P. Phys. Status Solidi A, 2009, 206, 391-408.
-
Takahashi, K. et. al., "DNA preservation using diamond chips", Diam. Relat. Mater. 2003, 12, 572-576.
-
Vadym N. Mochalin, Olga Shenderova, Dean Ho & Yury Gogotsi, "The properties and applications of nanodiamonds", Nature Nanotechnology 7, 11-23 (2012)
-
Igor Ahronovich, Andrew D. Greentree & Steven Prawer, "Diamond photonics", Nature photonics 5, 391-405 (2011)
