BME100 s2018:Group8 W0800 L5
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
OUR TEAM
LAB 5 WRITE-UPPCR Reaction ReportThe pre-lab reading was an extremely helpful guiding tool throughout this lab. It was used for reference multiple times during the procedure. Our group did understand the difference between the first and second stop on the pipettor. The first stop is used to collect the sample, and the second stop is to dispense it. The second stop adds a puff of air when expelling the sample in order to ensure it gets all of it out. After the reaction, the eight tubes did appear to have the same amount of liquid due to the measuring precision of the micropipettor. However, after the reaction was completed and the solutions were transferred, there was still a small amount of liquid left, which we just collected by doing another round of pipetting to ensure we got all of the sample. We did not change our labeling scheme, such that it was kept consistent throughout the lab. Fluorimeter ProcedureImaging set-up
-Inactivate the flash -Set ISO to 800 or higher -Set white balance to auto -Set exposure and saturation to the highest setting -Set contrast to the lowest setting
Placing Samples onto the Fluorimeter
Data Collection and AnalysisImages of High, Low, and Zero Calf Thymus DNA
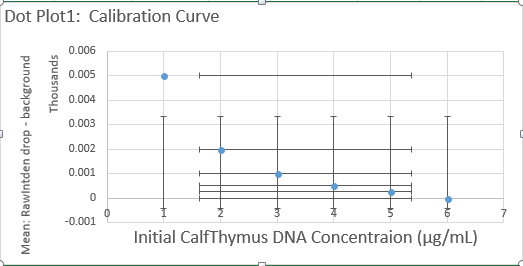
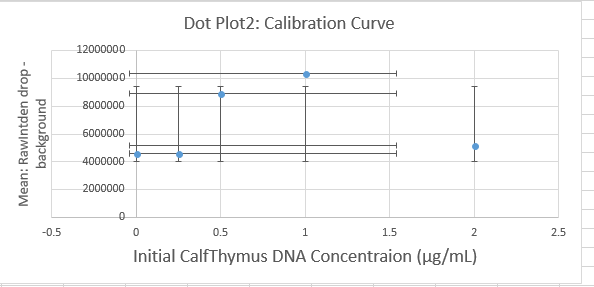
Calibrator Mean Values
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Images of Our PCR Negative and Positive Controls, respectively.

|

|
PCR Results: PCR concentrations solved
| PCR Product TUBE LABEL | MEAN (of RAWINTDEN DROP - BACKGROUND) | "PCR Product Concentration (µg /mL) | ||
|---|---|---|---|---|
| (Step 5 calculation)" | Total Dilution | "Initial PCR Product Concentration | ||
| (µg /mL) | ||||
| (Step 6 calculation)" | ||||
| Positive | 13358929 | 1.56 | 12 | 16.98 |
| Negative | 2956032 | -1.65 | 12 | -21.76 |
| Patient 1-1 | 6110412.7 | -0.63 | 12 | -7.56 |
| Patient 1-2 | 529585431 | -0.7 | 12 | -11.45 |
| Patient 1-3 | 6290890.7 | -0.64 | 12 | -6.34 |
| Patient 2-1 | 187006989 | 3.78 | 12 | 43.67 |
| Patient 2-2 | 141222345 | 2.45 | 12 | 23.78 |
| Patient 2-3 | 158333673 | 2.59 | 12 | 32.54 |
PCR Results: Summary
- Our positive control PCR result was 16.98 μg/mL
- Our negative control PCR result was -21.76 μg/mL
Observed results
- Patient 58297 : This patient had bright images, indicating that there was an extensive reaction between the DNA and the SYBER green. These images resembled those of the positive control. This makes sense since our calculated values were 43.67μg/mL, 23.78μg/mL, and 32.54μg/mL, which are values close to that of the positive sample.
- Patient 86632: This patient had dark images, indicating that the reaction between the DNA and the SYBER green was minimal. These images resembled those of the negative control. This makes sense since our calculated values were -7.56μg/mL, -11.45μg/mL, and -6.34μg/mL, which are values close to that of the negative sample.
Conclusions
- Patient 58297 : This patient was positive. This conclusion was reached because all three of the patients samples were calculated to have DNA concentrations in the positive range. These numbers were large, indicating that the DNA concentrations in the PCR samples were high, which means that DNA was replicated in large amounts in the PCR reaction. Moreover, these samples resembled the positive control, indicating that the disease primers were able to successfully bind to the disease positive patient.
- Patient 86632 : This patient was negative. This conclusion was reached because all three of the patients samples were calculated to have DNA concentrations in the negative range. These numbers were low, indicating that the DNA concentrations in the PCR samples were low, which means that the DNA replication did not occur. This sample resembled the negative sample, and since both samples did not light up, they are both negative.
|}