BME100 s2018:Group6 W1030 L1
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | |||||||||||||||||
|
OUR TEAM
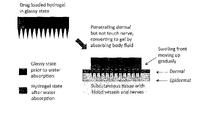
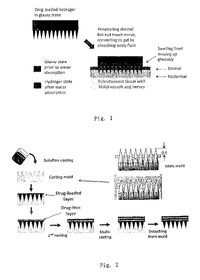
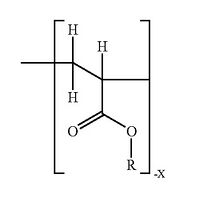
LAB 1 Needs IdentificationHealth Care Issue & SolutionHypothyroidism is also called underactive thyroid and it is a condition that affects more than 3 million people in the US. This condition is caused when the thyroid gland doesn’t produce enough thyroid hormone which can disrupt heart rate, body temperature, and metabolism.[3] Since the thyroid gland can’t make enough thyroid hormone, the body starts slowing down. Some of the symptoms include: feeling colder, tire more easily, dry skin, forgetfulness and depression. However, these symptoms aren’t the main diagnostic tool. In order to know for sure, a blood test for TSH is used to diagnose hypothyroidism.[10] There is no cure for hypothyroidism, but it can be controlled. It is treated by replacing the hormone that the body cannot make. T4 replacement restores the body’s thyroid hormone levels. [6] After diagnosed, a person is treated with the medicine levothyroxine and is instructed to take it daily at the same time, preferably in the morning 30 minutes to an hour before eating. [1] The dose of the medicine depends on the patient’s age, weight and the severity of hypothyroidism. Many patients find taking levothyroxine by mouth to be an inconvenience. Our solution is to create a small transdermal patch with poly-lactide-co-glycolide polymer microneedles that will deliver the medication to patients straight to their bloodstream. The dosage will be prescribed to patients from their doctors when they get their check-up. Then, they will be able to pick up their patches from any pharmacy. There will be a thousand of these tiny microneedles that are about as thick as a human hair. These microneedles will be made from biocompatible and biodegradable polymer material which will not only control the drug distribution, but will also be cost effective. They are made out of maltose, carboxymethylcellulose, amylopectin, poly (methylvinylether/maleic anhydride), sodium hyaluronate, chondroitin sulphate/dextrin, sodium alginate, and hydroxypropyl cellulose.[9] Plus, these swellable microneedles are made of hydrogels and contain a reservoir for the drug that can be used for prolonged delivery. Levothyroxine will be encapsulated within the needle and reservoir and then when put to the skin, will release and be absorbed by the blood stream. [9] And since the needles are made of polymers, they too will be dissolved by the skin.
Customer ValidationPhysicians: Physicians would contact us to validate our device because they are the ones who will provide the dosage to the individuals who need the device. 1. Endocrinologists 2. Dermatologists 3. Family Physicans Organizations: Organizations will provide our device with a validation that the device works and is safe to use for the public. 4. American Medical Association 5. Food and drug administration 6. World Health Organization 7. US Health and Human Services Individuals who suffer from hypothyroidism with any of these symptoms can validate our product to verify that it works the way it should. 8. low/high body temperature, tiredness, dry skin, forgetfulness/impaired memory, depression, weight gain/loss, elevated blood cholesterol level, thinning hair Providers: Providers validate our device by having the consumers pick up the patch from their local pharmacy after the patients physician prescribed the correct dosage. 9. Pharmacies Payers: The payers help validate our device by providing assistance to the consumers in terms of paying for the device. 10. Unitedhealth Group 11. Anthem 12. Aetna 13. Cigna 14. Humana 15. Kaiser Foundation Purchasers: The purchasers are in the same or similar business as our device, so they have been in the industry for a longer period of time. We could use this to our advantage to validate our device from companies that have experience, and maybe they could buy our company out. 16. Apotex 17. Abbvie inc. 18. Noven 19. Johnson & Johnson 20. Allergan Customer Adoption Risks: Medication needs to be specific and controlled, or else the patient might experience other problems.
Competitors
[3], [4]
IP PositionUS9320878B2 [13] Assignee: Tuo Jin File Date: October 7, 2008 Title: Phase-transition polymeric micro-needles Summary: Product is a system of phase-transition microneedles (PTM) made of a hydrophilic polymer. The microneedles are strong enough to penetrate the epidermis of the skin in a dry state. Once the needles have penetrated the epidermis they begin to swell. PTM are able to be removed without leaving anything behind. The desired substance for “injection” can be added to the polymer solution before molding without denaturing.
US7384650B2 [11] Assignee: Agile Therapeutics Inc. File Date: November 24, 1999 Title: Skin permeation enhancement composition for transdermal hormone delivery system Summary: The Transdermal Hormone Delivery System, THDS, is useful for the controlling of fertility and/or as a therapy for range of diseases and conditions that can be treated with a potent delivery of progestin and estrogen hormones, more so progestin, levonorgestrel. The THDS is composed of a backing layer, an adjoining adhesive polymer matrix forming a sufficient amount of at least the hormone progestin. Delivery of the hormone is enhanced by at least one or more skin permeation enhancing substance existing in a pre-determined amount. The Transdermal Delivery System is capable of administering an effective daily dose of hormones such as; progestin and estrogen, via skin contact surface area of less than 20 square centimeters.
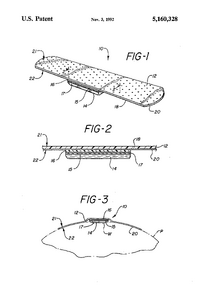
US5160328A [12] Original Assignee: NDM Acquisition Corp File Date: August 7, 1999 Title: Hydrogel bandage Summary: The bandage is made up of a first side and a second side and multiple layers including a backing layer which forms the first side of the substrate and an adhesive layer which comprises the second side of the substrate. A hydrogel layer is over the second side of the substrate. A polyurethane hydrogel material is used that which is principally used for absorbing bodily fluids, e.g., discharge from a wound. Also, a multitude of support layers may be interpolated between substrate and hydrogel layer to yield supplementary structure.
Fundability Worksheet ScoringCustomer Validation= 1, This number was given to us since we could not get specific customer validations. Competition= 2, We gave this score because there are no current microneedle patches specifically for patients of hypothyroidism that are readily available. So, if we introduce this product, we will not have too much competition. Even though there are competitors, clinical and technological improvements still need to be made. IP Position= 1, Since microneedle technology is still fairly new, we gave IP position a score of 1. There are some microneedles out in the market, but no patent applications have been filed for our specific type. Because of this, the patentability for our product is fairly weak.
Sources1. Bruno, Gwen. “How Soon After Taking Thyroid Medication Can I Eat?” LIVESTRONG.COM, Leaf Group, 14 Aug. 2017, [1]. 2. Chakera, Ali J, et al. “Treatment for Primary Hypothyroidism: Current Approaches and Future Possibilities.” Drug Design, Development and Therapy, Dove Medical Press, 22 Dec 2011. [2] 3. Mayo Clinic Staff. “Hypothyroidism (Underactive Thyroid).” Mayo Clinic, Mayo Foundation for Medical Education and Research, 6 Dec. 2017, [3] 4. Lécuyer, Manon, et al. “Clinical Efficacy and Safety of Transdermal Methimazole in the Treatment of Feline Hyperthyroidism.” U.S. National Library of Medicine, Feb. 2006, [4] 5. Padula, C, et al. “Innovative Formulations for the Delivery of Levothyroxine to the Skin.”International Journal of Pharmaceutics, U.S. National Library of Medicine, 8 May 2009, [5]. 6. “General Information/Press Room.” American Thyroid Association, [6] 7. Manon Lécuyer, et. al., “Clinical efficacy and safety of transdermal methimazole in the treatment of feline hyperthyrodism.” NCBI, 2006 Feb: 131-135. [7] 8. Park JH, et al. “Polymer microneedles for controlled-release drug delivery.” NCBI, 2006 May. [8] 9. Polymer Solutions News Team, “Polymer Microneedles Developed for Vaccine and Drug Delivery.” Polymer Solutions. 19 December 2013. [9] 10. Terrie, Yvette, "Hypothyroidism: What Every Patient Needs to Know." Pharmacy Times, 13 Jan 2011. [10] 11. US7384650B2 “Skin permeation enhancement composition for transdermal hormone delivery system." Google Patents. December 2006. [11] 12. US5160328A “Hydrogel bandage." Google Patents. December 2006. [12] 13. US9320878 “Phase-transition polymeric microneedles." Google Patents. Fall 2008. [13]
| |||||||||||||||||