BME100 s2018:Group5 W1030 L5
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | |||||
OUR TEAM
LAB 5 WRITE-UPPCR Reaction ReportOur experience with the pipetting went well. We all took turns to pipette the samples in the desired capsules, and got a feel for the tedious nature of sample pipetting. The pre-lab reading helped us to know what to expect from the lab, as well as knowing key terms and definitions within the lab. Being familiar with the material is definitely an advantage when dealing with a lab such as PCR Reaction. The first and second stop on the pipette was a little tricky at first, but we all got a hang of it. While using this instrument, it takes a great amount of dexterity as well as focus to perform at a high level. The final reactions did not all have the same amount of liquid in the end. There was no liquid left in the end of the capsules with the DNA and PCR reaction mix. No changes to the labeling were necessary. Fluorimeter ProcedureImaging set-up Placing Samples onto the Fluorimeter
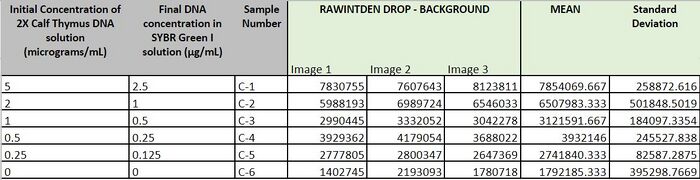
Data Collection and AnalysisImages of High, Low, and Zero Calf Thymus DNA
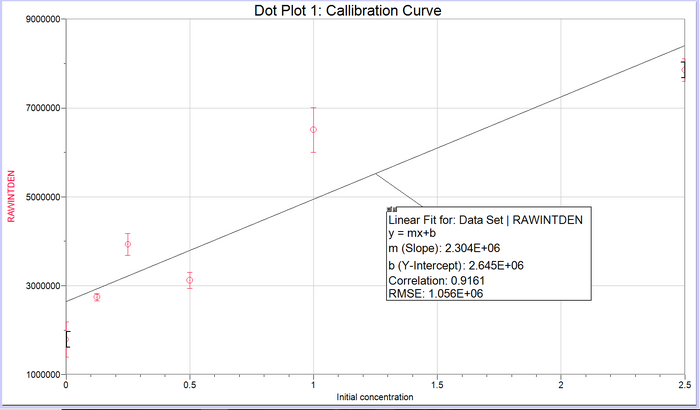
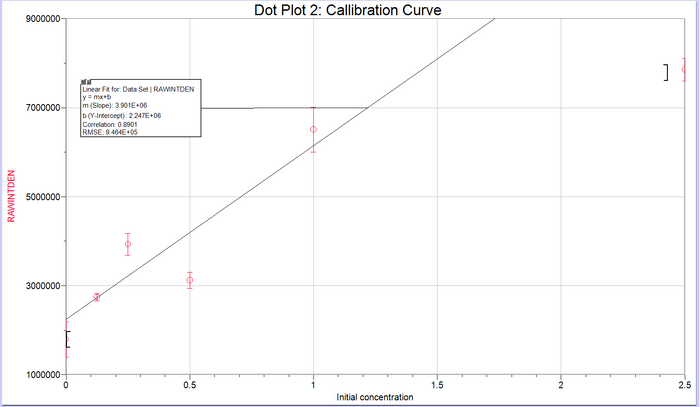
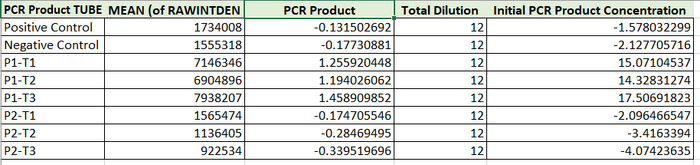
Calibration curves Images of Our PCR Negative and Positive Controls PCR Results: PCR concentrations solved
The images of this patient's DNA clearly showed fluorescence.
The images of this patient's DNA clearly showed no fluorescence. Conclusions
Because the positive control was faulty (no fluorescence) The patient DNA cannot be compared to it. However, the clear difference in fluorescence between patients indicates that this patient tested positive.
As mentioned above, the control values are inconclusive, but when compared to the first patient, this one appears to test negative.
| |||||