BME100 s2018:Group5 W0800 L1
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | |||||||||||||||||||||||||||||||||||||||||||||||||
|
OUR TEAM
LAB 1Health Care IssueBreast Cancer Breast Cancer is the leading cancer for new cases and top 5 for all cancers for deaths. It is reported that in the USA, about 12.4% women will develop breast cancer and about 40 thousand women are expected to die in the year 2018. Currently in the USA, more than 3 million women have had treatment or currently getting treatment in 2018. Many women are blindsided with breast cancer as more than 3 quarters of the women with breast cancer have no history due to genetic mutations. The sooner that women find about breast cancer, the better as the stages and the mutations will multiply and the ability to cure the breast cancer will be very difficult. Stage 1 survival rate is close to 100% and stage 4 is only a quarter of that. This proves to show that the sooner that people get diagnosed and treated immediately for breast cancer, the survival rate is much better. The current way of diagnosing breast cancer is the feeling of lumps, mammography and biopsy, which all can be done by a doctor. If there is a better way in diagnosing and having the early detection in breast cancer, the lives that can be saved is substantial.
The device that can be made to have early detection is to have a saliva swab to determine the proteins that are present when breast cancer is present. The swab and the device will provide an at-home diagnosis and plan of action if it does come positive. The swab will collect the patient’s saliva and then will place it back in the device to be collected and tested to see if the patient has breast cancer. The container of the device will take the saliva and test to see if the proteins are present. This device will decrease the amount of appointments to doctors and spend more and more money, with a chance of getting false results. To have this device be present, the amount of people that could buy it can be any women in the world that has a risk or don’t think they have a risk of breast cancer.
Customer ValidationPatients: Physicians: Purchasers: Providers: Payers: Why Choose Our Product These potential customers would be willing to validate our product because often times mammograms have false negatives, false positives and radiation from the testing that is harmful to patients. Our at home breast cancer testing kit will mitigate these false results by testing for the patients 15-3, tumor suppressor oncogene 53 and oncogene c-erbB-2 proteins.
Competitors
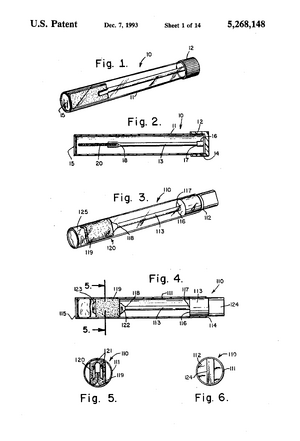
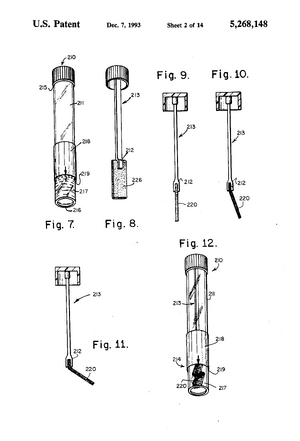
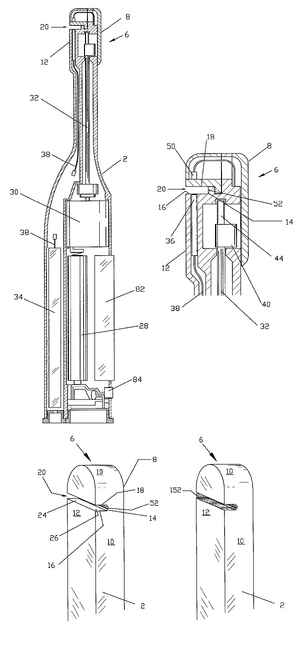
Existing PatentsSaliva sampling device and sample adequacy system (US5268148A) File Date: 1990-12-18 This patent is for the invention of a device that collects and mixes saliva in immunoassay tests. It includes a collection container, a saliva collector and a sample container. Handheld diagnostic device with renewable biosensor (US7314453B2) File Date: 2001-05-14 This patent is for an at home bodily fluid diagnostic device. It can draw in the fluid sample, mix it with a liquid reagant, and analyze it using a microprocessor. Method of diagnosing and monitoring malignant breast carcinomas (US6294349B1) File Date: 1999-03-01 This patent outlines the method for detecting breast cancer using proteins and antigens in saliva. Biomarkers such as cancer antigen 15-3, tumor suppressor oncogene protein 53 and oncogene c-erbB-2 are found in elevated levels in the saliva of women with breast cancer. Saliva test for early diagnosis of cancers (US20050106642A1) File Date: 2003-11-19 This patent outlines the use of proteomic cancer markers in saliva for early diagnosis of several types of cancer. Based on these biomarkers, it can measure presence and severity of cancer using an ELISA test.
Fundability Worksheet
Justification We gave competitors a score of 2 because the other diagnostic methods all have flaws and none compete with the at home convenience of our product, but the competition is well-established. We gave IP position a score of 2 because although there are a few patents related to our product, there are none for a at home breast cancer saliva test. The other patents are the foundation for our device, not necessarily conflicting.
References1) https://www.mayoclinic.org/diseases-conditions/breast-cancer/diagnosis-treatment/drc-20352475 2) http://www.breastcancer.org/symptoms/testing/types/ultrasound
| |||||||||||||||||||||||||||||||||||||||||||||||||