BME100 s2018:Group3 W1030 L5
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | |||||
OUR TEAM
LAB 5 WRITE-UPPCR Reaction ReportOur experience with pipetting was mixed all around. One was experienced with pipettes since they volunteered at a local clinic, another was new to the concept so it was rather difficult at first trying to remember the order of pipetting. The rest of us had been introduced it in a lab from another class. The pre-lab was useful because we were able to refresh on the idea and how it was used. It is easy to get mixed up and we did not want that because it could potentially ruin the samples. The difference between the two stops was what step you were on obtaining the samples. The first stop is to collect the samples at the preferred setting and if one were to go all the to the second stop while collecting the sample, then the sample would come back into the pipette rather the tip. The second stop is used while delivering samples by completely expelling everything in the tip. The final reaction did have the same amount of liquid at first but after close observation, there was a drop or two left in the sample tubes. Although the mircopipette is extremely accurate, it is known on not getting every single drop. The labeling scheme was kept the same to avoid confusion throughout the lab. Fluorimeter ProcedureImaging set-up
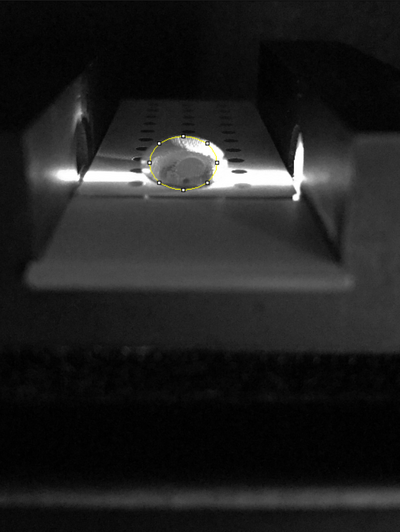
Placing Samples onto the Fluorimeter
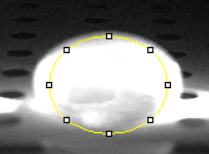
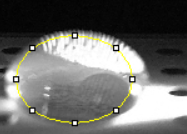
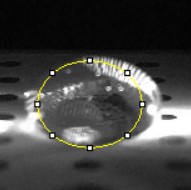
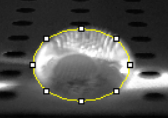
Data Collection and AnalysisImages of High, Low, and Zero Calf Thymus DNA
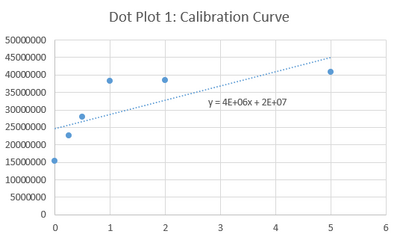
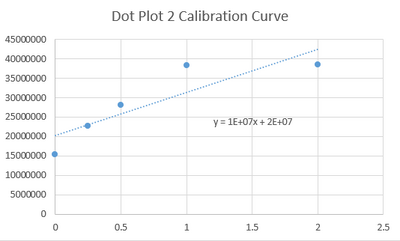
Calibration curves
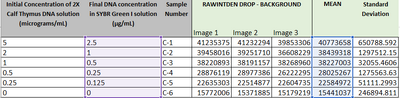
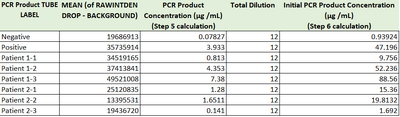
PCR Results: PCR concentrations solved
| |||||