BME100 s2018:Group3 W0800 L1
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | |||||
|
OUR TEAM
LAB 1 Needs IdentificationHealth Care IssueThe health care issue we are focusing on is Osteoarthritis, which is also called the “degenerative joint disease” and occurs when the cartilage or cushion between joints begin to wear away leading to swelling and or stiffness (1). Osteoarthritis can target any joint in the body, but it is most commonly found in the joints of the knees, hands, hips, and spine. Patients often suffer from joint tenderness, and a loss of flexibility -a lack of a full range of motion in joint. This is due to the deterioration of the slick cartilage, and a combination of an inflamed synovial membrane, and a reduced joint space. If the cartilage is completely destroyed, one may be left with bone rubbing on bone. Current medical treatments include acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs) , and or duloxetine - commonly used as an antidepressant (1). These are only meant to relieve the pain the patient would experience. The current surgical procedures that treat Osteoarthritis are comparatively invasive, and include among cortisone injections, realignment of the bones, and joint replacements (1). Impact of Issue on Society Solution Our solution would be a neural implant that would send electrical stimulations to the vagus nerve, and block the production of inflammatory proteins such as tumor necrosis factor (TNF) and prostaglandins that result in pain, warmth, and swelling. The implant would respond to a biological marker of arthritis, cartilage oligomeric matrix protein (COMP) in the bloodstream, and would release stimulation accordingly. By regulating the release of these proteins in a routine-like manner, the immune system could be regulated, and the patient could experience a reduction in pain during therapy or recovery.
Customer Validation1. On The Mend On The Move, 480-354-2911 http://onthemendpt.net/arthritis-rehab/ 2. Center for Athletic Performance, https://rebuildingchampions.com/arthritis-physical-therapy-exercises-symptoms.html 3. Daniel Choi, Pain Management Doctor, 5425 E Bell Rd # 115, Scottsdale, AZ 85254, (480) 991-3005 http://www.arthritis.org/ 12. Arthritis Today Magazine http://www.arthritis.org/arthritis-today-magazine/ 13. Arizona Pain Treatment Centers, 602) 833-4364 http://arizonapaintreatmentcenters.com/arizona-pain?_vsrefdom=cpc-google&gclid=EAIaIQobChMIwMup9Lzf2AIVFGV- Ch0CzAcZEAAYASAAEgLXxPD_BwE 14. Advanced Pain Management, (602) 899-PAIN http://advancedpainmanagement.com/ 15. Brigham and Women’s Hospital, 75 Francis St, Boston, MA 02115 https://www.azarthritisclinic.com/ 17. Johns Hopkins Arthritis Center, 5501 Hopkins Bayview Circle
Competitors
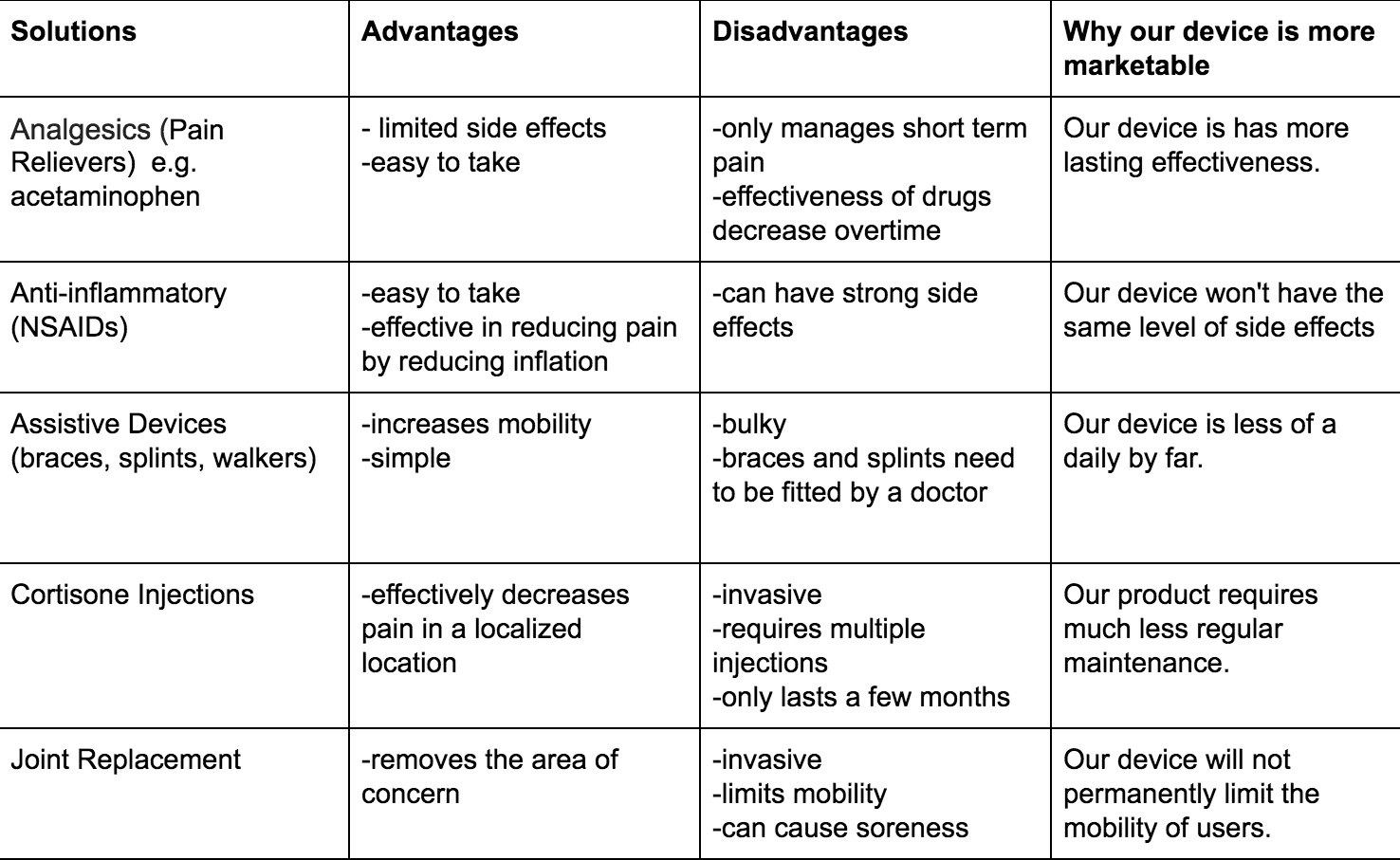
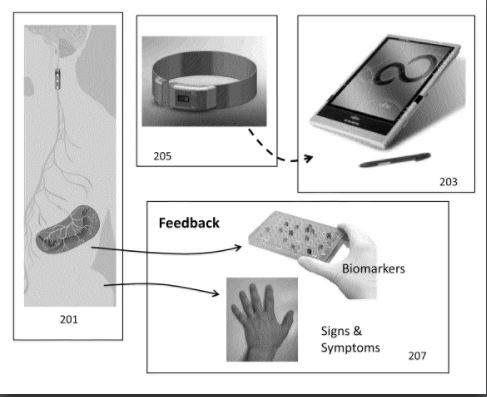
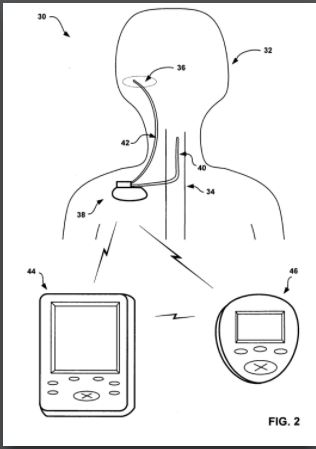
IP PositionPatent 1: Prescription Pad for Treatment of Inflammatory Disorders Prescription pad for treatment of inflammatory disorders, ZITNIK, Mar 3, 2011. The following is a patent for a cuff that goes around the vagus nerve that is surgically implanted in the patient. Once it has healed it sends information wirelessly to an electronic tablet that can keep track of the status of the implant as well as control the stimulation of the cuff. The prescription pad is then charge by a traditional charging module. The amount of stimulation will be entirely controlled by the doctor who will have access to input “prescriptions” into the pad. The doctor will get information from biomarkers in the patient and will use that information to determine the dose; therefore, the actual patient will not be able to accidentally over or under treat themselves.The controller would control the intensity of the stimulation the patient would receive. This device is designed to be mainly used for inflammatory disorders. Patent 2: Implantable Therapeutic Systems Including Neurostimulation Circuits, Devices, Systems and Methods Implantable Therapeutic Systems Including Neurostimulation Circuits, Devices, Systems and Methods, Scott Armstrong et. al, Nov 26 2011. This patent consists of two neurostimulation devices that will be connected and an array of neurostimulators that are controlled by an external controller. It is connected by the external device with an electrode on each end of the device. The neurostimulators contain non-volatile memory and respond to communications using identification codes. A polymer connector is attached to the first implantable stimulator which would form a neurostimulator array. Functions are performed by integrated circuits which have an input current rating from about zero to about five milliamps. The external controller (the control system) receives communications from the internal device, and allows the internal device to proceed with its function; Therefore, the external control system is composed of software and hardware that allows the control function. There are several different models for the process of stimulation including coils. This device does not target any specific disease and is simply a design for a nerve stimulation device and therefore is a very broad therapy.
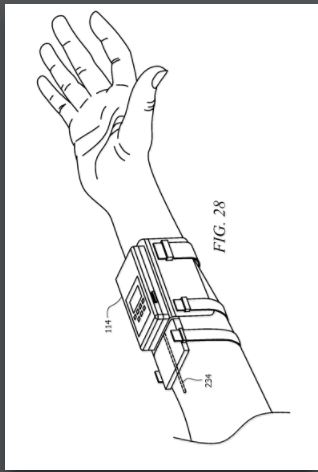
Treating inflammatory disorders by electrical vagus nerve stimulation, Jared Huston and Kevin Tracey, Aug 10, 2006. This patent includes a device to stimulate the vagus nerve using voltage to treat the inflammatory cytokine cascade. A teflon-coated silver electrode .003 inch in diameter is wrapped around the nerve. Stimulation is performed using the STMISOC stimulation adapter (a Biopac system). This device is designed to be used on an array of conditions, but focuses on the anti-inflammatory potential of nerve stimulation. The vagus nerve is stimulated by a signal voltage from 0,01 Volt to 1 Volt. However, this device is not effective if the patient suffers from asthma or cystic fibrosis. The half-life of TNF suppression by electrical vagus nerve stimulation was between two and three days, which is long-lasting compared to the pharmaceutical alternatives.The device can be implanted onto any surface of a nerve that branches off the main vagus nerve, and can be used to target a variety of inflammatory diseases. Patent 4:
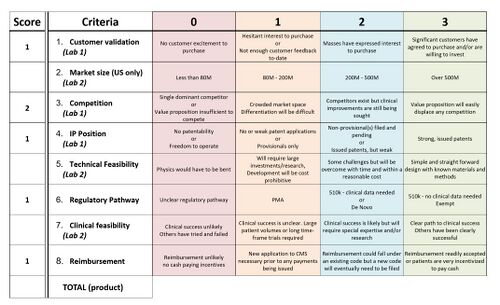
Fundability Worksheet Scores
Competitors IP Position
References1. "Osteoarthritis." Mayo Clinic,https://www.mayoclinic.org/diseases-conditions/osteoarthritis/diagnosis-treatment/drc-20351930. 2. Ben-Menachem, E., Revesz, D., Simon, B. J. and Silberstein, S."Surgically Impanted and Non-invasive Vagus Nerve Stimulation: A Review of Efficacy, Safety and Tolerability." European Academy of Neurology, vol. 22, no. 1, 2015, pp. 60–68. doi:10.1111/ene.12629. 3. Breedveld, F.C. "Osteoarthritis- the impact of a serious disease."Rheumatology, vol. 43, no. 1, 2004, pp- i4-i8. doi:10.1093/rheumatology/keh102. 4. "Osteoarthritis." U.S. Department of Health and Human Services,https://report.nih.gov/NIHfactsheets/ViewFactSheet.aspx?csid=55. 5. Koopman, F.A., Schuurman, P.R., Vervoordeldonk, M.J., Tak, P.P."Vagus Nerve Stimulation: A New Bioelectronics Approach to Treat Rheumatoid Arthritis?"Best Practice & Research Clinical Rheumatology, vol. 28, no. 1, 2014, pp. 25-35. doi:10.1093/rheumatology/keh102. doi: 10.1016/j.berh.2014.10.015 6. Manno, Rebecca L. “Osteoarthritis Treatment Information.” Arthritis Information, Johns Hopkins University, 11 Dec. 2011, www.hopkinsarthritis.org/arthritis-info/osteoarthritis/oa-treatments/#pharm. 7. “Osteoarthritis Treatment.” Www.arthritis.org, Arthritis Foundation , www.arthritis.org/about-arthritis/types/osteoarthritis/treatment.php. 8. Foltz-Gray, | By Dorothy. “Surgery: Why Wait? Why Not?” Www.arthritis.org, Arthritis Foundation , www.arthritis.org/living-with-arthritis/treatments/joint-surgery/candidates/waiting-surgery.php. | |||||