BME100 s2018:Group2 W0800 L6
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | ||||||
OUR COMPANY
Our Company: DNAid
LAB 6 WRITE-UPBayesian StatisticsOverview of the Original Diagnosis System With a total of 22 patients being tested each group of students tested two different patients. When testing patients for the disease associated SNP the work in each group was divided into two parts. One part was to prepare samples for imaging and taking the pictures of the prepared samples. The other part of work involved using ImageJ to analyze the pictures taken. To reduce error in results, three samples of each patient were taken. Three images were taken of each sample to find a more reliable average of each unique PCR sample. Additionally, positive and negative controls were used to validate results. The class found most of the patients to have negative results. Five patients were found to be positive, one patient was inconclusive, and two patient's results were not recorded at all. What Bayes Statistics Imply about This Diagnostic Approach
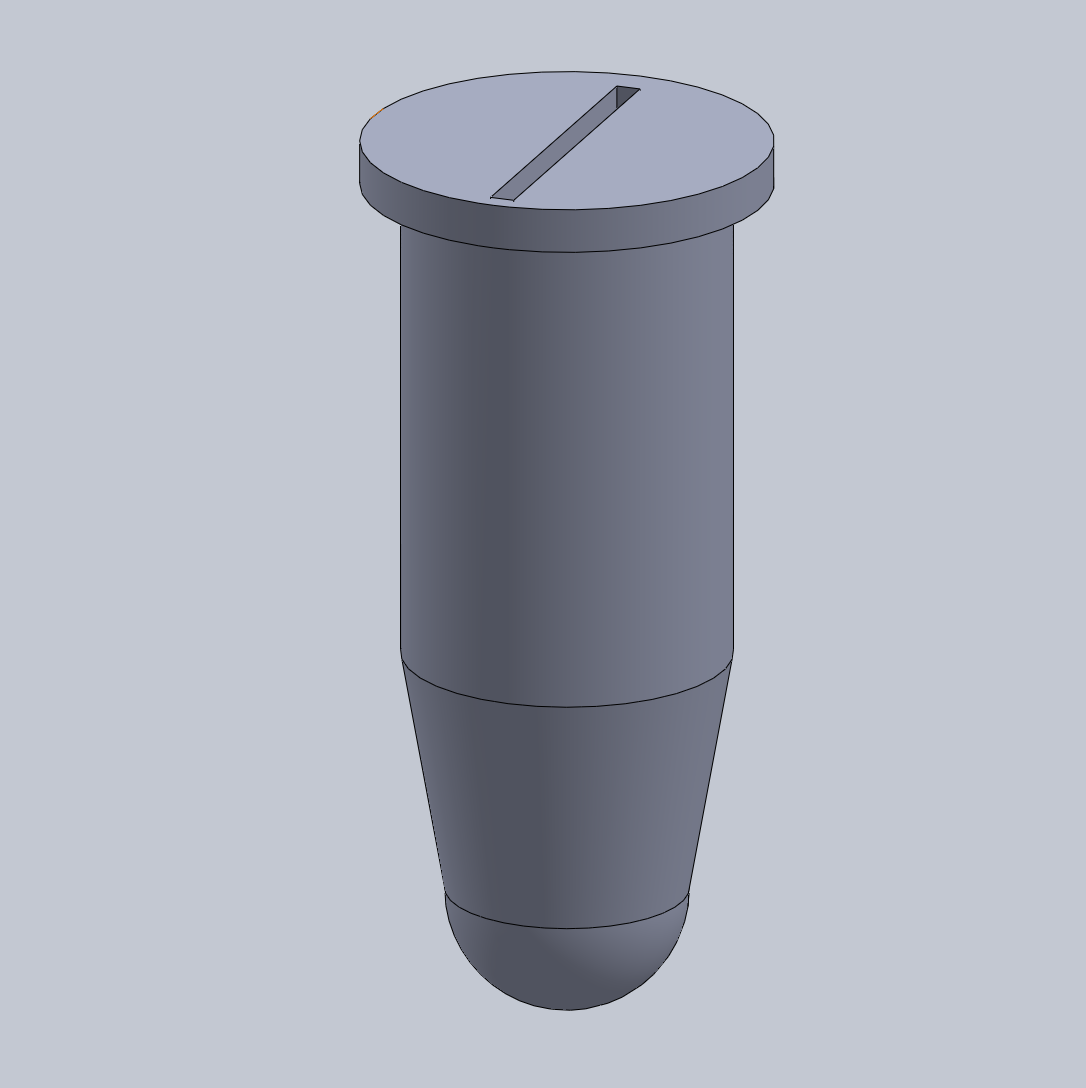
Intro to Computer-Aided Design3D Modeling Our team decided to use SolidWorks for the design of our new test tubes. SolidWorks is a powerful computer software that allows for the creation of 3D objects. The software allows for detailed model creation and has a lot of advanced tools to create realistic, 3D-printable objects. Our team's experience with SolidWorks was mostly positive, however we encountered a few struggles throughout the process of designing our new product. All members of our group have Mac computers and because the SolidWorks program tends to render and run slowly on Mac, it took a lot of time and effort to design our product. Additionally, we attempted to use the existing test tubes in order to create the 3D model, but it proved difficult making the model off of the actual object as opposed to a specification sheet. Consequently, our group decided to look for a specification sheet online in order to create the best 3D model possible for our idea.
The design for our test tubes is very similar to the existing design except the lid will utilize a pinch-to-open system where instead of snapping the lid on and off, the user will simply need to pinch the top and the specialized slit will open to allow the user to insert a micropipette tip into the tube and extract or expel solution. The design will remove the need to secure lids to the top of the tube as the new design will always be sealed until pinched.
Feature 1: ConsumablesOur PCR kit will include specialized PCR test tubes that will have a pinch-to-open lid. This new design will make it easier to access the materials inside the test tube as the user will not have to fiddle with the lid of the PCR test tubes and also maintain sanitation of the sample. The newly designed tubes will be of standard size in order to fit inside current PCR machines. The kit will not include other consumables such as the slides, pipettor, reagents, etc. as these are of standard dimensions. Feature 2: Hardware - PCR Machine & FluorimeterOur kit will continue to use existing technology for the PCR Machine and Fluorimeter processes. First, after the samples are properly mixed in the PCR test tubes, they will be placed within the PCR machine to undergo the standard PCR heating and cooling cycles. Then the tubes will be placed within the fluorimeter apparatus. Images will be captured and analyzed using the standard procedure.
| ||||||