BME100 s2018:Group11 W0800 L6
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | ||||||||
OUR COMPANY
Medivices  LAB 6 WRITE-UPBayesian StatisticsOverview of the Original Diagnosis System The results from each team were reported and used to calculate Bayesian statistics. While 22 patients were tested, Team #9 was unable to submit results on time and thus patient #55307 and patient #75503 were disregarded during the final statistical analysis. Out of the 20 patients included, 19 were diagnosed (the PCR results of patient #35518 were deemed inconclusive). Our group encountered few problems, however the fact that each team executed the experiment slightly differently may have influenced final results.
Overall the PCR test results aligned with the team's conclusion in the 90th percentage for both positive and negative results. Given how close to 100% both calculations 1 and 2 were, the PCR test proved to be reliable. Tests yielding negative results proved to be slightly more reliable than the positive mainly due to the sample size of the negative results. Out of the total sample size, 70 percent tested negative compared to only 25 percent of the sample size testing positive. Having a larger pool of samples would allow for more reliable test conclusions. Conclusion three gave about a 75% chance that if you are positive you will get the disease. Conclusion four was more reliable giving about 85% chance to not get the disease if the result was negative. The confidence of the Bayesian Statistics is effected by the population tested and the results found can be effected by the population used. Overall PCR testing proved to be a somewhat reliable way of diagnosing patients.
Intro to Computer-Aided Design3D Modeling
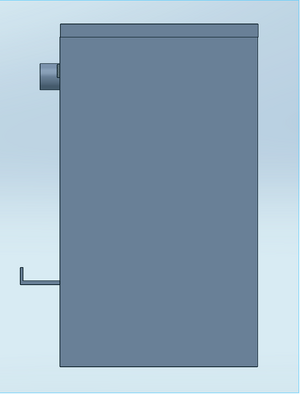
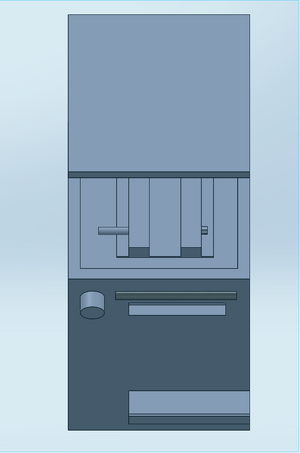
Our device was designed to solve one systematic problem with the original fluorimeter apparatus. During the experiment a smartphone was used to capture images of the droplets. Since the droplets had to be in a dark environment, a light box was used to cover the fluorimeter and the camera, meaning that each time a picture was taken the camera had to have a timer set and the box had to be repositioned over the system before that timer went off. This process was extremely tedious--it had to be done three times for each droplet. Indeed, since the apparatus was repositioned before each image, it likely affected the accuracy of the final results. To resolve this hassle, our design implements a camera holder on the outside of the box. Capable of adjusting the height with a basic crank mechanism using a knob, a simple ledge positions the phone camera precisely to capture images at the right distance from the droplet. The box has an open hole just for the phone camera. A combination of the phone and a piece of fabric hanging over it (attached via velcro strip) cover the rest of the hole, blocking light from entering the box. In addition, the box is designed with a rubber bottom to keep it from slipping when the phone is adjusted. Unlike the original fluorimeter, the top of our design is removable instead of the front. Made to attach magnetically, a removable top allows the drops to be easily added to the glass plate without having to move/adjust the entire apparatus. With this new design the whole system is connected. With the exception of the actual PCR droplets, no aspect of the design requires repositioning. Since the phone is placed in a specified slot, there is no need to measure and remeasure the distance between the phone and the slide. In other words, our design improves upon the original by keeping the experimental environment fixed abd removing the variability between trials.
Feature 1: ConsumablesOur consumables kit will include slides that will fit in our fluorimeter (size 2.5cm x 7.5cm), SYBR green solution in our newly designed darkened tubes, buffer, PCR mix , and plastic tubes with labels and the desired amount of primer in them. Major weaknesses we encountered were the labeling system of the tubes and using foil to keep the SYBR Green dark. To combat these issues we designed label stickers that can be put on the tubes. These will come with positive and negative stickers as well as stickers with sample and trials numbers. It will also include colors to help further organize the tubes. This is shown in the picture below. We found the foil covering the SYBR green tubes was not an efficient way to do so. To fix this issue we also designed a dark tube to not allow light to get to the SYBR green. Feature 2: Hardware - PCR Machine & FluorimeterAlthough the Flourimeter system and the PCR machines functioned well enough to provide reliable results, there were flaws that could be improved to make the process more efficient. Adjusting the height of the fluorimeter apparatus was the most inefficient aspect of the process. Stacking plastic boxes to adjust for height created an unstable platform as well as caused less accuracy in flushing the camera to the droplets. The cell phone stand also created issues. Long skinny phones such as the Samsung Galaxy 8 proved difficult to remain stable. The stand would also slide into to different positions after handling the phone to send and take pictures, requiring constant readjustment to ensure a consistent distance between the phone and the droplets. The system also did a poor job at keeping the fluorimeter concealed from light.The box's lid that covered the fluorimeter did not close all the way, sometimes affecting the quality of the images. The Open PCR machine's weaknesses were tied to its long heating process. The machines requirement of large PCR tubes and inefficient heat containing lid assisted in its unnecessary lengthy process. To address these issues, the Fluorimeter system will incorporate a more efficient smartphone holder where the smartphone will remain in a fixed position until completion of the experiment, this will eliminate any issues with realignment and refocusing of the camera. The phone itself will be height adjustable to ensure the stability of the apparatus and to create a simplified way to flush the camera and droplet. With the phone in a fixed position on the face of the system, a removable magnetic lid will be on top. The removable magnetic top will create a tight seal, concealing the fluorimeter completely from light. To make a more time efficient PCR machine, a seal will line the bottom of the lid to better contain the heat during the PCR process. Also, the machine will allow a much smaller sized PCR tube to fit in the machine to increase the speed of the reaction.
| ||||||||