BISC220/S12: Mod 2 Lab 6
Background Information
Lab 5: Phenotypes of Secretory Mutants
Lab 6: Analyzing Secretion Defects by Western Blotting
Lab 7: Probing and Detecting the Western Blot
Media Recipes
Analyzing Secretion Defects by Western Blotting
In today’s lab you will have two main tasks:
- Scoring the results of the ss-HIS4 reporter gene assay
- Performing the initial steps of a Western blot, using a different reporter, to determine which forms of a normally secreted protein accumulate in the two sec mutant strains
Protein Maturation in the Secretory Pathway
Proteins that travel through the secretory pathway usually undergo several types of modifications before they reach their mature forms. The signal sequence that targets secreted proteins for co-translational insertion into the ER is usually cleaved off by a signal peptidase just after that part of the protein enters the ER, even before the entire protein has been synthesized by the ribosome (Waters, 1988). Second, most secreted and plasma membrane proteins are modified by the addition of carbohydrates in a process called glycosylation. Branched chains of sugar molecules (oligosaccharides) are initially added to proteins in the ER in a process called glycosylation. Some trimming of these chains occurs before proteins leave the ER and proceed to the Golgi. In the Golgi, additional carbohydrate modifications often occur. As a result, multiple, differently glycosylated forms of a particular secreted or cell surface protein are present in a cell at any one time, as different molecules of the protein travel through the secretory pathway. Since they have different molecular weights, these various forms of a protein can be separated by SDS-PAGE and, if an antibody is available, can be detected by Western blotting.
Pre-pro-α-factor
In this experiment you will perform a Western blot to detect the various forms of a normally secreted protein, α-factor, that are present in the wildtype and sec mutant yeast cells. Alpha-factor is a yeast mating pheromone. Yeast can exist as both haploid and diploid cells. Haploid yeast come in two mating types (MAT): a and α. MATa yeast secrete a pheromone called a-factor, while MATα cells secrete α-factor. These pheromones allow yeast cells to sense cells of the opposite mating type that are in close proximity and to initiate events that prepare them to mate (i.e. to fuse into a diploid cell). We will talk more about yeast mating factors in the cell signaling portion of the course, but for now, the important thing is that they are secreted proteins that must travel through the ER, the Golgi, and subsequent compartments before being released at the cell surface by exocytosis.
The mature, secreted form of α-factor is a polypeptide of only 13 amino acids, but it is synthesized as a larger precursor protein that is processed to its mature form in several glycosylation and proteolysis steps. Using the nomenclature that is generally applied to secreted polypeptides derived from larger precursors (e.g. many hormones), the form of α-factor that is translated by the ribosome is referred to as “pre-pro-α-factor” (pp-αF). This form includes the ER signal sequence and other “spacer” sequences that are eventually removed to generate the mature α-factor polypeptide. The term “pro-α-factor” (minus the “pre-“) would typically refer to an intermediate form of the final product, after cleavage of the signal sequence (which usually occurs just after a protein’s translocation into the ER) but prior to removal of spacer sequences.
An antibody raised against pre-pro-α−factor would be expected to recognize all forms of the protein as it progresses through the various glycosylation and proteolysis steps in its processing sequence. The molecular weight of the unglycosylated, translated form (including the signal sequence) is approximately 18.6 kDa. In the ER, N-linked core oligosaccharides are added to three sites in the protein, causing its molecular weight to increase significantly; the fully modified ER form of pp-αF is 26 kDa (Julius, 1984). Many secreted proteins undergo additional carbohydrate modifications in the Golgi, but glycosylation of pp-αF is completed by the time it leaves the ER. As pp-αF moves through the Golgi and into the secretory vesicles that bud from the Golgi, endo- and exo-proteolytic steps eventually produce the mature, secreted form of α-factor, reducing it to 3.4 kDa (see Fig. 1).
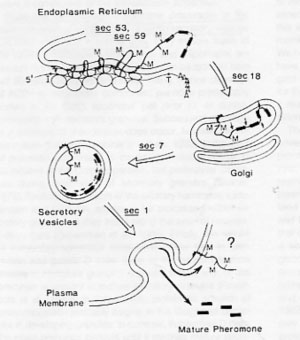
Figure 1. Processing of pre-pro-α-factor as it passes through the secretory pathway (Julius, 1984).
Using pre-pro-α-factor to investigate the steps blocked in sec18 and sec61 mutant yeast
For the Western blot analysis you will probe for precursor forms of α-factor in WT, sec18, and sec61 yeast cells that were either grown continuously at room temperature (RT, 25°C) or grown at RT then shifted to 37°C for one hour. Note that all of these strains are haploids of mating type alpha (α) (MATα): If they were mating type a (MATa) haploids or if they were diploid strains, they would not produce α-factor! Recall that the sec18 and sec61 strain each have a temperature sensitive defect in a different step in the secretory pathway. The Western blot will allow you to determine the molecular weight of the form of α-factor that accumulates in each of these strains due to the block in secretion. The WT strain will serve as a comparison control and a negative control lysate of a wildtype MATa strain that doesn’t express any alpha factor will be supplied.
What should happen to α-factor in a cell expressing it that has a normal secretory system?
References
Julius, D., Schekman, P., and Thorner, J. (1984). Glycosylation and Processing of Prepro-α-Factor through the Yeast Secretory Pathway. Cell 36:309-318. (posted on lab e-reserve conference)
Karp, G. (2005). Cytoplasmic Membrane Systems: Structure, Function, and Membrane Trafficking. In: Cell and Molecular Biology: Concepts and Experiments, 4th ed. John Wiley & Sons, Inc., Hoboken, NJ. p. 279-333.
Waters, M.G., Evans E.A, Blobel, G. (1988) Prepro α factor has a cleavable signal sequence. Journal of Biological Chemistry 263(13): 6209-6214.
SDS PAGE Protocol
Microsoft Word File: Media:SDS PAGE Protocol.doc
- Label your six tubes of yeast cells with your initials and make sure the cells are thawed. To each tube of cells, add 200 µl of SDS sample buffer (10% SDS, 25% glycerol, 250mM Tris pH6.8, 500mM, DTT, 0.5% BPB) and “1 scoop” of glass beads. The sample buffer contains SDS and a reducing agent to ensure that the proteins are fully denatured. You will find six microfuge tubes, each containing 1 scoops of glass beads, at your bench so you can just pour the beads from these tubes into the screw-capped tubes that contain your yeast cells. The beads are to insure that the tough yeast cell wall is broken in step 2. Your instructor will prepare a tube of negative control Mat a wild type yeast cells by adding 600 µl of sample buffer and 3 scoops of glass beads and processing as in step 2 & 3. These cells will be available at the instructor’s bench for everyone to use.
- Make sure the caps are tightly closed. Process the samples at room temperature in the FastPrep bead beater for 20 seconds (speed = 6.0). Your instructor will assist you in loading and unloading the samples from the machine. Yeast cells have a tough cell wall, so the glass beads are necessary to lyse the cells.
- Centrifuge 10 minutes at maximum speed in a microcentrifuge.
- Label 7 microfuge tubes according to the chart below (Lanes 2-8). Transfer 50 µl of each lysate you prepared and 50 µl of the Mat a yeast lysate that should not contain alpha factor (negative control), found on the instructor’s bench, to the correct labeled microcentrifuge tubes, taking care to avoid the beads and cellular debris at the bottom of the lysis tubes.
- Boil all 7 samples for 3 minutes at 95°C in a heating block. Place a plastic cap holder on each tube to prevent it from popping during heating and spraying your sample everywhere. When you take your samples out of the heating block, carefully vent each tube by opening the caps very slightly (pointing awayItalic text from you) to allow the vapor to escape. Some liquid may spurt out—you can just wipe this off with a KimWipe™.
- The gels you will be using are 18% Tris-HCl polyacrylamide, which will slow the migration and help us visualize small forms of alpha factor. Each group will run one gel, but two groups can use a single electrophoresis apparatus. When you pipet your samples to load on the gel, draw from the top of the liquid, trying to avoid sucking up any stray glass beads. Load your samples as shown below.
- Run your gel for ~35 min. at 200 volts.
Gel Loading:
Lane 1 — 20 µl of pre-stained MW standards (do not need to be boiled)
Lane 2 — 20 µl of SEC+, 25°C lysate
Lane 3 — 20 µl of SEC+, 37°C lysate
Lane 4 — 20 µl of sec18, 25°C lysate
Lane 5 — 20 µl of sec18, 37°C lysate
Lane 6 — 20 µl of sec61, 25°C lysate
Lane 7 — 20 µl of sec61, 37°C lysate
Lane 8 — 20 µl of MATa lysate
Finishing the SDS-PAGE & Starting the Anti-pp-αF Western blot
Turn the power source controlling your gel electrophoresis to OFF. Remove your gel from the apparatus and separate the plates. Carefully cut off the wells at the top of the gel with a razor blade. Be careful not to tear the gel. Take it over to the blotting apparatus.
The holder for the blotting apparatus is color coded so the black side should end up facing the cathode (black electrode) and the clear side facing the anode (red). The sandwich of choice, therefore, will be made in the following manner, keeping all parts wet in the blotting buffer in the plastic container provided at your bench:
- Wear gloves when handling the nitrocellulose membranes. The membrane is white, and will be given to you sandwiched between two pieces of blue protective paper. Remove the top piece of blue paper. In pencil, label the top left corner of your piece of nitrocellulose (NC) with your initials. Be careful not to tear the membrane with the pencil tip. Pour some blotting buffer into your small plastic container. Immerse the NC in blotting buffer so that it is wet evenly.
- Place the blotting holder black side down in another plastic container after filling the container half full with blotting buffer.
- Open the blotting holder, wet one of the sponges with blotting buffer and place it on the black side of the holder.
- Wet 2 pieces of 3 mm paper and place them on top of the sponge.
- Wet your gel with blotting buffer in the small plastic container and place the GEL on top of the 3 mm paper. To make the left-most lane (Lane 1) of the gel come out on the left side of the blot, orient your gel so that Lane 1 (containing the stained MW ladder) is on the right side of the blot sandwich.
- Place your wet NC on top of the gel.
- Add 2 more pieces of moistened 3 mm paper on top of the NC. Use a broken plastic pipet like a rolling pin to gently “roll” out any air bubbles out from between the gel and the NC.
- Place a second moistened sponge on top of the 3 mm paper.
- Close the clear side of the holder, pushing the clasp down and along the top of the holder.
- Place the holder into the blotting tank so that the clear side faces the red pole and the black side faces the black pole.
- Two blots can be run in each tank. Place a frozen ice compartment into the tank. Fill the tanks so the buffer is up to the top of the gel. Connect the top of the tank tightly. Connect the power supply and run the blots at 100 volts for one hour. Be sure the stir bar is free to stir to keep the buffer cold. Watch the current. It should read 0.15-0.20 amps during the run.
- After an hour, disconnect the power and remove the holders. Remove the NC, rinse it with distilled water, and stain it with Ponceau S protein stain to see if the proteins were transferred properly to your membrane. Pour the 25ml provided of PonceauS onto your NC, placed in the small plastic container and rock back and forth for 15-30 sec. Pour stain back into 50ml conical tube (DO NOT DISCARD!). Rinse off the protein stain by pouring distilled water onto stained blot, rock, and discard water into sink. Look for approximately equal protein bands in all lanes loaded with cell lysates and look for presence of MW marker bands. Once you have determined that your transfer worked and that all lanes have approximately the same amount of protein, allow the membrane to dry a bit, then wrap it in plastic. Label it with your name and lab section. Store the NM in the refrigerator in a container provided by your instructor until the next lab.
Protocol: Finishing the Yeast Spotting Experiment
Microsoft Word File: Media:RESULTS for yeast secretory pathway REV 2010.doc
Scoring the results of the growth phenotype assay:
DO NOT refer to your table of predictions until you have scored your results.
Score growth in the results table according to the following relative scale:
++ , + , +/- , or – (NG)
Consider a spot of growth that is confluent (fills circle completely) as ++.
Rate growth as + if there are many or a few small visible colonies, but growth over the entire spot is not uniform.
Growth can be scored as +/- if there are tiny colonies that are clearly different from the film of cells left from the spotting procedure.
Be very careful not to consider the film of dead yeast cells left from the spotting procedure as growth. Ask your instructor for help if you are not sure how to make this distinction.
After you have recorded your results, compare your results to your predictions. Do they agree? If not, think about why you might see growth that you did not expect or vice versa. Which strains are “controls” and for what do the controls test? Temperature sensitive mutants have variable sensitivity and some strains may be more or less temperature sensitive than expected. Think about which nutrient genes are present or lacking in the plasmids pRSB204, pRSB203 and in the vector plasmid YEp24. Consider whether the gene product is a fusion protein and where in the cell that fusion protein is directed. Now think about how the proteins encoded by the plasmid interact with the SEC gene defects found in the yeast mutants. What differences do you see when comparing the 9 strains’ growth pattern in the same conditions (on the same plate) and what differences do you see when you compare each strain’s growth at different temperatures and on different media?
Now look at your results vs. your expectations. If you have unexpected growth (or no growth) where you expected to see colonies, try to come up with possible explanations for discrepancies. You may have time to begin this analysis while your proteins separate or during the transfer phase of the Western blot. Resolving the growth patterns with expectations may be a challenging exercise. Feel free to discuss your thoughts with your partner or others in the class, but make sure any work that is submitted for grading is entirely your own.