BISC209/F13: Pour Plates
Making Solid Medium Plates
1. Place the sterile Petri plates to be poured right side up in a laminar flood hood that has been disinfected (if many plates are to be poured, you may want to set them up in stacks of three).
2. Obtain a bottle of melted (45°-50°) sterile medium with ~1-2% agar from a hot water bath. Carefully wipe the adhering water from the outside of the bottle so that, when you tip the bottle, this non-sterile water will not run into the sterile plate. Light a Bunsen burner and properly adjust the flame. Remove the cap of the bottle as illustrated in Figure A-3a. Lightly pass the lip of the bottle through the flame to create a temperature differential that prevents dust from dropping into the bottle. The lip of the bottle should already be sterile from the autoclaving procedure.
3. Pour 10-15 ml of molten agar into each plate (Fig A-3b). Stop when the plate is 2/3 to 3/4 full. With practice you will learn how to judge the proper amount by eye. Be careful to prevent the drip on the lip from running down onto the contaminated outer glass. Let the medium cool and solidify without disturbing it. The lids should be off or ajar so that excessive condensation doesn't form on the inside of the lid.
4. When the medium has cooled to RT and is solid , cover the plates.
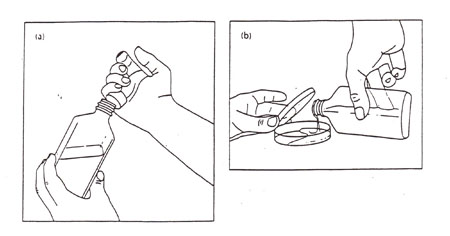
Figure A-3: (a) Removal of a bottle cap aseptically. Note how the rest of the hand is free to manipulate a pipette or plate lid. (b) Pouring agar into a plate. Note how the lid shields the agar from airborne contamination.
Preparing A Spread Plate
5. Use a cooled solid medium plate (such as those prepared above) and inoculate the center of the plate with a quantity of microorganisms in liquid culture or suspended in sterile liquid (usually ~100microliters) sufficient to yield between 30-300 CFU (colony forming units). If you don't know how concentrated your culture is, prepare several pour plates with different dilutions of your culture so that one is likely to yield this range of CFU.
6. Use a sterile glass spreader that has been flame sterilized and cooled or sterile glass beads to uniformly distribute the inoculum over the plate.
5. Carefully slide the plates to an undisturbed area for ~5-10 min to allow the liquid to absorb into the medium. Label the plates, invert and incubate until isolated colonies appear.