BIO254:AP
Introduction
An action potential is a wave of electrical activity carrying information within and between tissues. It is a brief, explosive change in membrane potential which goes from a negative to a positive potential. Action potentials are self-regenerating and occur spontaneously when the membrane is depolarized to a critical voltage called the threshold. Action potentials enable nerve cells to carry signals over long distances.
Action Potentials are Initiated by a Change in Membrane Potential
A neuron at rest maintains an electrical potential difference across its membrane. This difference arises from the separation of electric charges across the resistive membrane barrier. In the neuron, the major ions in play are potassium (K+) and sodium (Na+).
Membrane potential is determined by the equilibrium potential and relative permeabilities of the ions in the system. [K+] is higher inside the neuron while [Na+] is higher outside the neuron. This creates a driving force for K+ to go out of the cell and Na+ to come into the cell. At rest, the membrane is most permeable to K+; thus, the resting potential is closest to the equilibrium potential of K+ (~ -110mV). However, during an action potential, the permeability to Na+ is dramatically increased by the opening of Na+ channels. This depolarizes the membrane and brings the potential closer to the equilibrium potential of Na+ (~ +50mV).
An increase in ionic conductance in the membrane of the axon results in an increase in the action potential. The rising phase of the action potential is caused by an influx of Na+, while the falling phase of the action potential is caused by a later increase permeability to K+.
Resting Potential vs. Action Potential
The pattern or frequency of action potentials define a neuron's ability to transmit information throughout the nervous system. In contrast, when cells are at rest, or when there is a lack of electrical signaling, they maintain a resting membrane potential of approximately -60 to -70 mV. By convention, the potential outside the neuron is arbitrarily defined as zero, and the relative number of intracellular negative charges creates a potential difference across the plasma membrane; this is why resting potentials are expressed as negative values. The opening and closing of Na+ and K+ channels during an action potential make electrical signals an active process, yet membrane pumps and channels must also be active to maintain the resting membrane potential. To preserve the chemical and electrical gradients necessary for the initiation of an action potential, various membrane proteins must work to transport ions across the cell surface. The Na+/K+-ATPase enzyme is perhaps the most crucial membrane pump found in neurons; for each ATP molecule hydrolyzed, this enzyme extrudes three Na+ ions and intakes two K+ ions. Maintaining the membrane potential is energetically costly--up to 60% of a cell's energy or ATP supply may be used to bring the cell back to resting membrane potential following an action potential.
Differences between resting membrane and action potentials are apparent on the molecular level: while action potentials utilize voltage-gated ion channels, ATP-dependent pumps are used for resting membrane potentials. The two major ions that play a role in initiating and ending action potentials are Na+ and K+. However, Cl-, Ca++, Na+, and K+ may all help contribute to the resting membrane potential. While the membrane is at "rest," ionic species are not distributed evenly across the plasma membrane; Na+ and Cl- ions are more concentrated on the outside of the neuron while K+ and organic anions (proteins) are concentrated intracellularly. Action potentials disrupt this concentration gradient through sudden influx and efflux of Na+ and K+ respectively. Many neurodegenerative deseases such as epilepsy may stem from a neuronal inability to maintain the resting membrane potential, such that abnormal electrical signals fire spontaneously and irregularly. Hence, a better understanding of the mechanisms leading to action potentials as well as proper maintenance of resting potentials can provide insight into the causes of such disorders.
Characteristics of the Action Potential
1. Action potentials are triggered by depolarization. This is normally in the form of an external stimulus such as the stretching of a muscle or the firing of another neuron.
2. A threshold level of depolarization must be reached in order to trigger an action potential. This level is typically 10-20mV.
3. Action potentials are all-or-none events. Once an action potential is initiated, its amplitude is independent of the strength of the stimulus.
4. The amplitude and form of an action potential remain constant along the length of an axon. An action potential travels uniformly down the axon at a rate of ~ 10-100m/s. The speed depends upon the diameter and myelination of the axon.
5. At the peak of the action potential, the membrane potential reverses sign, becoming inside positive. The membrane potential transiently overshoots zero (called the overshoot). Also, during repolarizing, it becomes more negative than normal (called the undershoot).
6. After a neuron fires, there is an absolute refractory period during which it is impossible to trigger another action potential. This period is ~ 1ms. Thus the maximum firing rate of a neuron is ~ 1000 action potentials per second.
The following is a link that demonstrate all the above!
- Named link:
Phases and Landmark Points of an Action Potential
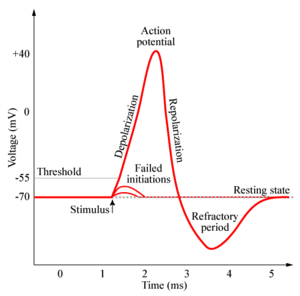
1. Threshold. Before an action potential begins, the initial depolarization must reach a threshold of ~ 10-20mV above resting potential, when the inward Na+ current exceeds the outward K+ current. The net influx of positive charges depolarizes the membrane potential and leads to opening of more voltage-gated sodium channels. This positive feedback cycle continues depolarizing the membrane potential.
2. Rising Phase. Voltage-dependent sodium channels open, thus increasing Na+ permeability. There is a large driving force on Na+ which pushes Na+ into the neuron. The membrane depolarizes towards the equilibrium potential of Na+.
3. Overshoot. The period during which the membrane potential is above zero.
4. Peak. The highest level of depolarization reached before the falling phase.
5. Falling Phase. Repolarization of the membrane occurs when a second class of K+ channels opens, increasing the permeability of K+ to exit the cell. In addition, Na+ channels are time-dependent and inactivate, reducing the permeability of Na+. This reduces the inward current of Na+.
6. Undershoot. The period during which the membrane potential is below resting potential. The membrane is hyperpolarized.
7. Refractory period. Action potentials cannot be generated for ~ 1ms after an action potential is fired. This is a result of Na+ channel inactivation.
Propagation of Action Potential Along Myelinated Axons
In myelinated axons, the action potential appears to jump along the axon in a process known as saltatory conduction. The action potential jumps from one unmyelinated Node of Ranvier to the next, being regenerated only at these nodes. This increases the conduction velocity without the need for a dramatic increase in axon diameter. The myelin decreases membrane capacitance, reducing the extent to which ions of opposite charge interact with each other from across the cell membrane and thereby increasing the speed at which the ions are conducted. However, this prevents the regeneration of action potentials because of the lack of ion channels in these areas. The Nodes of Ranvier contain many voltage-gated sodium channels and are spaced along the axon to allow for the regeneration of the action potential. The internodal length is such that the optimal balance between high speed of action potential conduction and robustness of the signal is achieved.
Propagation of Action Potential Along Unmyelinated Axons
In unmyelinated axons, when voltage-gated Na+ channels are opened by depolarizing one patch of cell membrane, Na+ ions enter the cell. Postively-charged Na+ ions attract negative ions away from adjacent membrane. Thus, positive ions travel down the axon. When the adjacent patch of membrane is depolarized, the voltage-gated Na+ channels open, repeating the cycle. An action potential is generated at each segment of the membrane.
Voltage-Clamp Technique in Action Potentials
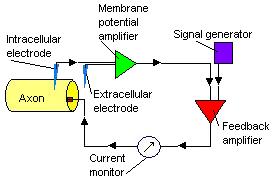
The voltage-clamp technique by Hodgkin and Huxley enables us to understand the ionic mechanisms in action potentials. From the voltage-clamp experiment, when there is a small depolarizing voltage, there is a brief outward capacitive current, a leakage current that persists for the period of the depolarization and then a brief inward capacitive current, before the ionic current returns to zero. When there is a larger depolarizing voltage, the initial brief outward capacitive current is larger in amplitude. This is followed by a brief inward leakage current and an outward leakage current that is maintained for the duration of the depolarization voltage. The inward Na+ channel and outward K+ channel is turned on sequentially by the depolarizing voltage and the two currents partially overlap in time. By using chemicals such as tetrodoxin to block Na+ channels and tetraethylammonium to block K+ channels, we can determine the currents belonging to Na+ and K+ channels. The voltage-clamp technique shows that Na+ channels turn on and off more quickly than K+ channels.
Voltage-Gated Sodium and Potassium Channels
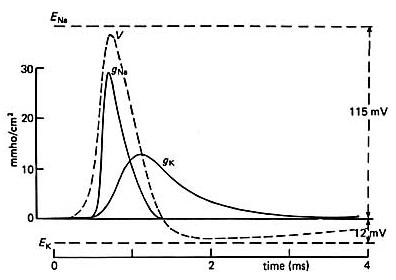
Na+ and K+ open when the membrane is depolarized. However, after some time, Na+ channels begin to close, causing inward current to decrease (known as inactivation), while K+ channels remain open. Voltage-gated Na+ channels have 3 states - resting, activated and inactivated. In the resting state, the activated gated is closed and the inactivated gated is open. In the activated state, the activation gate opens and Na+ flows through the channel. In the inactivated state, the inactivation gates close. By repolarizing, Na+ channels return to the resting state. A small interval between two depolarizing pulses result in a small increase in the conductance of Na+. However, a longer interval between the two depolarizing pulses result in a larger conductance of Na+ because more Na+ channels will be in resting stage when the second depolarizing pulse arrives.
Depolarizing the membrane causes Na+ channels to open quickly and an inward Na+ current to flow. The inward current flow causes further depolarization and more Na+ channels to open. The positive feedback cycle drives the membrane potential to the peak of the action potential, attributing to the rising phase of the action potential. The voltage-gated K+ channels are delayed in opening and the depolarizing state of the action potential results in an inactivation of the Na+ channels causing the action potential duration to be brief. The decrease in inward Na+ current and an increase of outward K+ current repolarizes the membrane.
Electrical Equivalent Circuit to Calculate Membrane Conductances
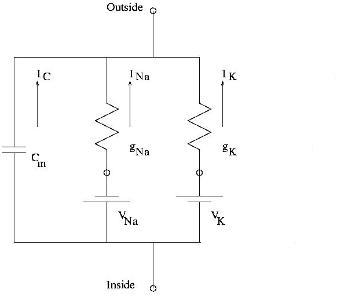
From Ohm's law, the current through X+ voltage-gated channel, where X+ = Na+ or K+,
[math]\displaystyle{ I_X = g_X(V_m - E_X) }[/math]
Rearranging, the conductance of X+,
[math]\displaystyle{ g_X = \frac{I_X}{V_m - E_X} }[/math]
[math]\displaystyle{ V_m }[/math],[math]\displaystyle{ E_X }[/math] and [math]\displaystyle{ I_X }[/math] are determined from voltage-clamp data.
Genes of Potassium and Sodium Channels
The genes of K+ and Na+ families come from a common family. They share several important domains and are quite similar. In Na+ channel molecules, three subunits are isolated - one large glycoprotein [math]\displaystyle{ \alpha }[/math] and two smaller polypeptides [math]\displaystyle{ \beta_1 }[/math] and [math]\displaystyle{ \beta_2 }[/math]. The [math]\displaystyle{ \alpha }[/math] subunit forms the aqueous pore of the channel, while the smaller subunits regulates the [math]\displaystyle{ \alpha }[/math] subunit.
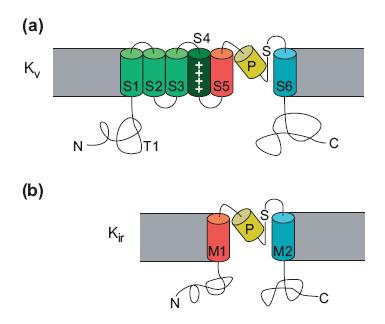
The charge in the S4 region influences the opening and closing of the voltage-gated Na+ channel. In the resting stage, the net negative charge inside the membrane attracts the positively charged S4 region inside the membrane. However, when the cell is depolarized, the change in electrical charge across the membrane drives the S4 region toward the extracellular face of the membrane, opening the activation gate. The K+ channel genes has one repeat in the [math]\displaystyle{ \alpha }[/math] subunit. However, to form a K+ channel, four [math]\displaystyle{ \alpha }[/math] subunits surround a central pore.
Major References and Recommended Reading
Bear, M.F., Connors, B.W., and Paradiso, M.A. Neuroscience: Exploring the Brain. (Lippincott Williams and Wilkins, Baltimore, Maryland, 2001).
Matthews, G.G. Cellular Physiology of Nerve and Muscle, Fourth Edition (Blackwell Science Ltd, United Kingdom, 2003).
Kandel, E.R, Schwartz, J.H, and Jessel, T.M. " Principles of Neural Science, Fourth Edition" (McGraw Hill, 2000)
Hodgkin, A.L., Huxley A. F., Katz B., "Measurement of Current-Voltage Relations in the Membrane of the Giant Axon of Loligo" (J. Physiol II6, 424-448, 1952)
Miller, C., " An Overview of the Potassium Channel Family" (Genome Biology, 2000)
Goldin, A.L., "Mechanisms of Sodium Channel Inactivation" (Elsevier, 2003)
Neher, E. "Ion Channels for Communication between and within cells" (Neuron 8, 605, 1992)
Hille, B. "Ionic Channels in Nerve Membranes", (Progress in Biophysics and Molecular Biology 21, 1-32, 1970)
Recent updates to the site:
List of abbreviations:
- N
- This edit created a new page (also see list of new pages)
- m
- This is a minor edit
- b
- This edit was performed by a bot
- (±123)
- The page size changed by this number of bytes
2 March 2026
1 March 2026
| 00:19 | CHIP:Talks diffhist +16 Gabor Balazsi talk contribs | ||||