BISC209/F13: Lab3: Difference between revisions
Tucker Crum (talk | contribs) |
Tucker Crum (talk | contribs) |
||
| Line 212: | Line 212: | ||
The data should be saved automatically in softmax pro, but please also save to our BISC209 folder on the desktop, labeling clearly with your group letter and the date. | The data should be saved automatically in softmax pro, but please also save to our BISC209 folder on the desktop, labeling clearly with your group letter and the date. | ||
Because the instrument computer is not networked, you will have to Save the data to a FLASH DRIVE. <BR> | Because the instrument computer is not networked, you will have to Save the data to a FLASH DRIVE to export it to your Sakai data file. <BR> | ||
Export the labeled data to the Flash drive | Export the labeled data to the Flash drive by clicking on the blue image of the plate in the upper left of the screen. From the menu that appears, click Export. Then Choose Plate1 and Output format PLATE raw (.txt). Click OK. Then SAVE AS with your site and date AND change the file type to .xls (not the default!). Save to the USB (D:) drive. | ||
Close the Spectromax software. DON'T SAVE! (You have already saved and exported adequately.)<BR> | Close the Spectromax software. DON'T SAVE! (You have already saved and exported adequately.)<BR> | ||
Shut off the SpectraMax spectrophotometer unless you know another group is waiting. DO '''NOT''' SHUT DOWN THE COMPUTER!!!<BR> | Shut off the SpectraMax spectrophotometer unless you know another group is waiting. DO '''NOT''' SHUT DOWN THE COMPUTER!!!<BR> | ||
Eject the Flash drive hardware properly by right clicking on the left image on the bottom of the computer's tool bar. If that doesn't work, go to Computer--USB (D:) Eject<BR> | |||
Remove Flash drive and take it to the lab computer facing the Spectromax and plug it into a USB port. | |||
<BR> | |||
'''DATA STORAGE:'''<BR> | |||
Open your team's Data folder from Sakai and download and open your group's Biolog data sheet. The file is called: BIOLOG-CMD_calc&GraphGroup__2013fa. <BR> | |||
Then EDIT: PASTE SPECIAL: TEXT the data carefully to your team's Excel spreadsheet making SURE that you are on the appropriate day (found in tabs on the bottom of the workbook). Note that each day that you collect data has a separate page in this spreadsheet.<BR> | |||
Copy and paste one column in the spectrophotometer 96 well format at time into your group's spreadsheet. After pasting column 1, 2 and 3 you will begin the next set of replicates (columns 4-6 then 7-9). <BR> | |||
'''This is a very important step because it aligns the replicate cells properly. '''<BR> | '''This is a very important step because it aligns the replicate cells properly. '''<BR> | ||
| Line 227: | Line 230: | ||
[[Image: Read2.jpg]] | [[Image: Read2.jpg]] | ||
When you have finished, | When you have finished, go to Finder and eject the Flashdrive from Devices. Wait for it to disappear. Please store the flash drive in the USB port in the computer on the Spectromax. If it is not there, send a message to the class to see if someone took it home by mistake and email your instructor. You can still transfer your data to your Sakai data folder if the disk is missing by reading off the data from the Spectromax computer to your open data Spreadsheet. (The 2 computer screens are close enough to allow that.) If you need to look at the Biolog template to see if you are transferring the appropriate cells you can pull it up from the Powerpoint intro for Lab 3 found in Resources in Sakai. It is crucially important that you align the right carbon source to its value (don't forget water) and get the right well numbers transferred to the appropriate position on the Worksheet DAY (1, 2,etc) in the template workbook. Note that the Template contains a separate worksheet (tabs on the bottom) for each day within the full workbook. Make sure that you fill out the appropriate day (DAY 0, 1, 2 etc). If you miss a day, leave that page blank. | ||
PRECAUTION: If you haven’t saved your data and another group reads their plate, your data will be overwritten and lost.<BR><BR> | PRECAUTION: If you haven’t saved your data and another group reads their plate, your data will be overwritten and lost.<BR><BR> | ||
DATA ANALYSIS:<BR> | |||
The Spreadsheet is pre-formatted with the calculations for these data. It includes the formulas to average replicate measurements each day and it will automatically subtract the background (readings in the water wells). There is a normalization for background that will be subtracted automatically (this threshold absorbance is 0.25 for each carbon source). We hope that including these pre-made calculations in the template will make your calculation of community metabolic diversity (CMD) and the data analysis of carbon source utilization patterns relatively uncomplicated. Please make sure you understand the EXCEL performed calculations. When you are sure that your data has been copied correctly into the appropriate template day, you will notice that the built in calculations will provide a final average absorbance reading for each substrate. | The Spreadsheet is pre-formatted with the calculations for these data. It includes the formulas to average replicate measurements each day and it will automatically subtract the background (readings in the water wells). There is a normalization for background that will be subtracted automatically (this threshold absorbance is 0.25 for each carbon source). We hope that including these pre-made calculations in the template will make your calculation of community metabolic diversity (CMD) and the data analysis of carbon source utilization patterns relatively uncomplicated. Please make sure you understand the EXCEL performed calculations. When you are sure that your data has been copied correctly into the appropriate template day, you will notice that the built in calculations will provide a final average absorbance reading for each substrate. | ||
Revision as of 20:23, 24 September 2013
Lab 3: Community Level Analyses of Richness & Co-operative & Competitive Behavior in a Soil Microbial Community
BACKGROUND
Because each type of bacteria in a soil community fills a unique niche and may play a slightly different role in nutrient cycling or contributing to soil structure, it is important to identify group members and to study them individually, when possible. However, it is equally important to study the cumulative functionality of the community.
The culture-dependent work at the soil microbial community level that we will set up today will have the goal of studying richness as functional metabolic diversity and as potential for co-operative & competitive community behavior. Microbial members of a soil community must both exploit and provide resources for the community in order for such an enormous number of individuals to survive and thrive. This balanced co-operation and competion requires diversity in metabolic capabilities. Soil microorganisms demonstrate extreme diversity in their abilities to alter raw materials into useable nutrients. Bacterial communities can be thought of as functional units comprising the sum of the metabolic properties of its members. Functional diversity in substrate utilization for both the anabolic and catabolic parts of their metabolism is of great importance, particularly if one is concerned about the long-term health of a habitat or soil community.
One of the reasons bacteria can exist in large numbers in a small area is because all of them might not compete for the same raw materials. A soil community can work co-operatively to share the “work” of processing available raw materials into forms that other members can use if they don't all synthesize the same metabolic enzymes; the enzymes a microbe makes often controls which nutrients a soil community member can use or process. Because soil bacteria and fungi are the microorganisms largely responsible for the turnover of plant material, changes in the numbers of these microorganisms can be related to changes in the organic matter content of soil and affect other non-microbial members of an ecosystem or habitat.
All bacteria require a source of carbon rich "food" and heterotrophic bacteria require it from organic compounds because they are unable to make carbon-carbon bonds. In a crowded soil community with millions of members competing from available organic materials to supply their carbon needs, it is advantageous to be able to use sources that are not useable by other members of the community or to be able metabolize a wide variety of carbon sources to allow survival when common sources become depleted or are unavailable. Diversity in carbon source utilization allows competition for carbon to become a co-operative strategy in the soil microbial community as a whole.
Make another Soil Extract and Serial Dilution
You will prepare another soil extract today from a new soil sample so that you can set up some tests for the prevalence of specific exoenzymes that have an important impact on the availability of nutrients for the soil community. You will start some measurements of metabolite cycling and utilization. We will assess, specifically, at the community level, how well your soil microbes interact co-operatively to use a variety of carbon sources and to secrete digestive exoenzyme that are able to process essential or commonly available nutrients into widely useable forms. The nutrients we have chosen to look at are phosphates, cellulose, and starch.
Our goal is to obtain quantitative evidence for richness of the microbial soil community and for co-operative behavior in the community. We will quantify this soil microbial community's ability to use a variety of carbon sources for its anabolic needs as CMD (community metabolic diversity). We will also assess the proportion of microbes in the community that can secrete enzymes to process phosphates into a soluble form, or to degrade cellulose or starch into simpler raw materials. The prevalence of those enzymes in the community can be seen as evidence for co-operative behavior as well as to serve as another measure of functional metabolic diversity.
Soil Extract Preparation:
Make one soil extract made for each sample site. Prepare the serial dilution in pairs.
- Using a freshly collected and sieved soil sample collected prior to lab process the soil as you did in lab 1. Weigh 1 gram of sieved soil using the top loading balance and add it to 100 mL of sterile water which you will find premeasured for you in a sterile flask on your bench.
- Swirl to mix--- don't use the magnetic stirrer yet.
- Pour this soil suspension into a clean blender jar. Use gloves when you place the cap on the jar, first making sure that the rubber gasket is properly positioned and the seal at the bottom is tight (otherwise it will leak!).
- Blend for 3 pulses of 10 seconds on and 10 seconds off.
- Pour all of the suspension back into the flask and add a sterile magnetic stir bar (on your bench).
- Place the flask on a magnetic stirrer at medium speed and mix for at least 15 min.
- Stop the stirring and let the soil settle until the larger particulate matter settles to the bottom. Not all visible particles need to settle, just the big stuff. When you take an aliquot from it, avoid the particulate matter in the lower portion.
- In making this extract, you have created a 10-2 or 1/100 dilution. This extract can also be described as a 1% (wt/vol) solution since there is 1 gram/100 ml.
Serial dilutions:
Each pair will prepare a series of dilutions to use in the community physiology profiling analyses.
- 4 large (18 X 100), sterile tubes with caps that can hold a volume of 10 ml and not spill it when vortexed
- 4 1ml sterile, disposable pipets and a blue pipet pump
- 1 10ml sterile, disposable pipet and a green pipet pump
- P1000 micropipet and sterile tips
- Bottle of sterile water
1. Label four large 18 mm sterile tubes 10-3, 10-4, 10-5, 10-6 until you reach the appropriate dilution series for your soil extract. (Carry out the dilutions until you are one dilution past the one that gave you 30-300 colonies in your plate count from Lab 1-2).
2. Pipet 9 ml of sterile water into each tube using a 10 ml sterile, disposable serologic pipet. (You may use the same sterile 10 ml pipet for all of the tubes if asepsis is maintained.)
3. Using a different sterile, disposable 1ml serologic pipet for each transfer, transfer 1ml of the 1% soil extract (1:100 dilution made of 1gram of soil) to the next dilution tube labeled 10-3; mix well by vortexing.
4. Using a new pipet, transfer 1ml of the 10-3 dilution to the tube labeled 10-4. Mix well. (Mixing 1ml of the 10-3 dilution with 9ml of sterile water makes a 10-4 dilution. )
5. Continue to transfer 1ml aliquots (after mixing well) from each dilution to the next dilution tube of water until you have carried the dilution to the last dilution tube.
- Prevalence of Exoenzyme producers able to digest Starch, Cellulose, and/or solubilize Phosphate
- Community Carbon Source Utilization Pattern and calculation of Community Metabolic Diversity in Carbon Source Utilization
Community Physiological Profile of Starch Digesters, Cellulolytic Bacteria, and Phosphate Solubilizing Bacteria: Background
Degradation of complex carbon containing materials such as leaves and other plant matter provides an important source of a wide range of organic molecules to heterotrophic members of a soil community. Cellulose and starch are two complex carbohydrates that can be important sources of carbon, if broken down to a simpler, useable form. Many, but not all, soil bacteria contain the enzymes necessary to play this important role. It is not surprising that bacteria and other microbes are able to digest molecules associated with plants since plant roots are always present in the soil and plant parts become part of the soil litter that adds organic matter.
Starch Hydrolysis: Starch is a common plant storage compound. It is a complex polysaccharide carbohydrate of sugar (glucose) molecules joined by glycosidic bonds. When the hydrolytic exoenzyme amylase is secreted, the starch in the surrounding environment will be degraded. Microbes capable of degrading starch extracellularly to small mono or disaccharide products allow nearby non-amylase producers in the community to take up the smaller digested products and to produce energy (ATP) from this abundant raw material.
Cellulolytic Activity: Cellulose is an organic compound with the chemical formula (C6H10O5)n. It is a polysaccharide consisting of several hundred to over ten thousand linear, linked β(1,4) D-glucose units. Cellulose is a primary structural component of the cell wall of green plants, many forms of algae and some other types of organisms. Some species of bacteria secrete cellulose to form biofilms. Cellulose is the most common organic compound on Earth comprising 40 to 60% of plant residues.
Some animals can digest cellulose with the help of symbiotic microorganisms that live in their guts. For example some termites contain flagellate protozoa in their hind guts that produce cellulases while other termites and cows rely on gut bacteria. Fungi are very good at breaking down cellulose, just consider the shelf mushrooms that help degrade fallen trees. Humans can digest cellulose to some extent, but cellulose consumed by humans as food is largely undigested and acts as a hydrophilic bulking agent for feces ("dietary fiber"). Soil microorganisms capable of catabolizing cellulosic material contribute to the carbon cycle and, ultimately, contribute to the release of CO2to the atmosphere--- making it available for re-uptake by plants. Cellulolysis is the process of hydrolytic break down of cellulose into smaller polysaccharides called cellodextrins or complete break down into glucose units. Because cellulose has a somewhat complex structure, cellulolysis is relatively difficult compared to the breakdown of other polysaccharides. The enzymes used to cleave the glycosidic linkage in cellulose are glycoside hydrolases. They include endo-acting cellulases and exo-acting glucosidases. Such enzymes are usually secreted as part of multienzyme complexes. Soil bacteria and symbiotic anaerobic bacteria (like the Cellulomonas group of bacteria) are among those known to produce and secrete these enzymes to benefit the whole community.
Phosphate Solubilizing Activity: Phosphorus is one of the major nutrients that limits plant growth. It is important in physiological activities such as photosynthesis, root development, cell division, and for efficient use of carbon substrates. Most phosphorus is found in an insoluble form; thus, the conversion of insoluble phosphorus into soluble forms that can be absorbed by plants is an important interaction between plants and phosphate solubilizing bacteria. The fact that phosphate solubilizing bacteria process phosphate extracellularly, means that these bacteria can meet their own needs for a useable form of this essential nutrient AND they allow other bacteria in the community to find phosphorus in a useable post-processed form.
Because soil is, in some ways, a non-renewable resource, the activity of the microbial population in maintaining soil quality is of paramount importance and a diverse and rich population of phosphate solubilizing bacteria play a crucial role in the improvement and repair of soil health destroyed by years of environmental abuse.
Community EXOENZYME Prevalence:
Nutrient Agar control
Starch hydrolysis prevalence
Cellulose digestion prevalence
Phosphate Solubilizing prevalence
Protocol for all exoenzyme prevalence assays:
Refer to your standard plate count calculations (Lab 2). You will use the dilution that gave you 30-300 CFUs when you evaluated your plate count in Lab2 AND one dilution higher and one dilution lower. Use those 3 dilutions to inoculate the media described below. Using three dilutions (with 2 replicates for each dilution) will ensure that at least one dilution of each medium will have colonies of the appropriate density to be accurately counted and evaluated next week.
1. Colony Count on Nutrient Agar (NA) General Purpose Medium as a Control
(0.3% Beef extract, 0.5% Peptone, 1.5% Agar at pH 6.6- 7.0 at 25°C) is used to determine comparative number of total culturable bacteria:
Using your P200 micropipet and sterile tips, dispense 100µl of the appropriate soil extract dilution onto each of two pre-labeled Nutrient agar plates. Use a sterile, disposable spreader to evenly distribute the diluted soil extract all over each culture plate. Repeat for the other two appropriate dilutions. Incubate all the plates at RT until visible colonies have appeared. Check the cultures after 24 hours to make sure they aren't close to being overgrown and again at 72 hours. Move the culture plates to the cold room before they become overgrown. Continue to check your plates every few days until you transfer them to the cold room. Your goal is to have between 30-300 countable colonies on one of the dilutions.
2. Starch Medium (2.5%(wt/vol) soluble starch in Nutrient Agar) is used to determine the % of amalyase producing, starch digesting culturable microbes when compared to the total number counted in NA:
Using your P200 micropipet and sterile tips, dispense 100µl of one of the soil extract dilutions into the center of each of two pre-labeled starch agar plate at the appropriate dilutions. Choose the dilution that is expected to result in 30-300 cfu/g soil. Use a sterile, disposable spreader to evenly distribute the extract aliquot. Repeat for a soil extract dilution that is 10 fold more and one 10 fold less dilute. Incubate all plates at RT until mature, visible colonies appear. Move the culture plates to the cold room before they become overgrown.
3. Cellulose Congo Red Agar Medium is used to determine the % of cellulolytic microbes (those producing cellulase and able to digest cellulose)
Using your P200 micropipet and sterile tips, dispense 100µl of one of the soil extract dilutions into the center of each of the pre-labeled cellulose congo red agar replicate plates. Choose the dilution that is expected to result in 30-300 cfu/g soil. Use a sterile, disposable spreader to evenly distribute the extract aliquot. Repeat for a soil extract dilution that is 10 fold more and one 10 fold less dilute. The ashed, acid-washed cellulose and the Congo red in the medium will allow enumeration of cellulose-digesting bacteria in soil. Incubate all plates at RT until mature, visible colonies appear. Move the culture plates to the cold room before they become overgrown.
Cellulose Congo Red Agar:
0.05% K2HPO4; 0.025% MgSo4; 0.188% ashed, acid washed cellulose powder; 0.02% Congo red, 1.0% Noble Agar, 0.2% gelatin, 10%(vol/vol) sterile soil extract(10.5% air-dried, sieved soil that's autoclaved twice, allowed to settle, and the supernatant filtered); final pH 7.0 - 8.0.
Reference: Hendricks, C.W., Doyle, J.D., Hugley, B. (1995) A new solid medium for enumerating cellulose-utilizing bacteria in soil. Applied and Environmental Microbiology 61, 2016-2010.
4. Phosphate Medium (Pidovskaya medium [PVK]) is used to determine the % phosphate solubilizing microbes (those producing phosphatases) in a soil community:
Using your P200 micropipet and sterile tips, dispense 100µl of one of the soil extract dilutions into the center of the two pre-labeled Phosphate medium (PVK) replicate plates. Choose the dilution that is expected to result in 30-300 cfu/g soil. Use a sterile, disposable spreader to evenly distribute the extract aliquot. Repeat for a soil extract dilution that is 10 fold more and one 10 fold less dilute. Incubate all plates at RT until mature, visible colonies appear. Move the culture plates to the cold room before they become overgrown.
Pidovskaya Medium Modified (Nautiyal, 1999)
1% glucose; 0.5% Calcium Phosphate [Ca3(PO4)2; 1% MgCl2.6H2O; 0.25% Magnesium Sulfate (MgSO4.7H20);0.2% (NH4)2SO4; 0.25% KCL; 0.0025% BromoPhenol Blue; 1.5-2% agar
References: Pikovskaya, R.I. 1948. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Microbiologia 17, 362-370>
Pranjal Baruah (2007)Isolation of phosphate solubilizing bacteria from soil and its activity. Available at: Biotechindia.files.wordpress.com/2007/12/isolation.pdf.
Community Carbon Source Utilization Profiling:
Carbon Source Utilization
One type of metabolic diversity that we will assess in our investigation is physiological diversity in carbon source utilization.
You have learned in other courses about the importance of carbon fixation by autotrophic photosynthetic plants. The inability to make carbon-carbon bonds and, therefore, to utilize carbon dioxide as a carbon source is problematic for heterotrophic species including humans and all other animals. Fortunately there are bacteria that, like plants, are autotrophic and photosynthetic, although many others are heterotrophic, like us. Unlike us, however, bacteria are extremely diverse in the types of carbon sources they can use metabolically. Soil bacterial communities both compete and co-operate in utilization of available sources of essential, useable carbon. The health and longevity of the community is dependent on a continuous supply of useable carbon for all its members. Your investigation on community carbon source profiling will attempt to quantify some of that co-operation and competition.
Carbon source patterns using BIOLOG™ Community Level Physiological Profiling (CLPP)
Observing patterns of substrate utilization can provide evidence of functional diversity of a microbial community. Additionally, understanding how metabolic substrates can be used in soil communities can help us understand the stability or flexibility of an ecosystem. Carbon sources are crucial anabolic raw materials for heterotrophic microbial growth. Microbes vary enormously in their ability to make use of carbon in different forms. Using direct inoculation of soil community samples into a variety of carbon substrates will allow us to study and measure potential community carbon source utilization and provide us with evidence for our hypothesis that soil community diversity allows co-operative as well as competitive interactions.
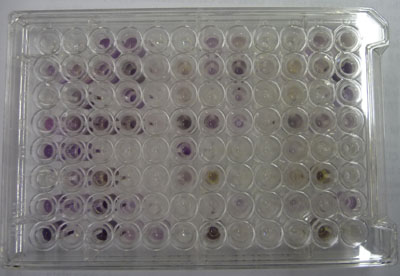
In community level physiological profiling (CLPP) the metabolic properties of individual bacteria in the community contribute to the total metabolic capacity of the community. Mixed environmental samples are inoculated directly into the single carbon source wells of microtiter plates followed by spectrometric quantification of growth. If one or more microbes in the community can use a particular carbon substrate, the metabolism of that carbon source is accompanied by a capture of electrons from water-soluble colorless Tetrazolium salts (WTS) to become reduced purple formazans. WST-1 and in particular WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (MTT), are reduced outside of the cells. They combine with an electron mediator (phenazine methosulfate (PMS)), to yield a water-soluble purple product called formazan that can be measured spectrophotometrically at 590nm. The color development is additive and directly proportional to the metabolism of each carbon source so the development of forazan can be followed over time. The intensity of purple color as a pattern in the wells is used to determine a characteristic reaction pattern classed a metabolic footprint. We will use the patterns to determine community metabolic diversity (CMD). For these measurements to be meaningful, it is important to control for number of microbes, incubation time, and other microenvironmental factors as well as the requirement for saturating substrate and indicator concentrations. There are 31 carbon sources available on a BIOLOG plate. This set of substrates is far from exhaustive. However, these substrates were chosen for variety and to simulate the range of carbon sources commonly found in the soil, either from root exudates or from decomposition of organic molecules.
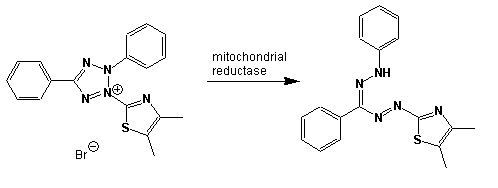
Scheme showing the reduction of MTT to formazan. Image created by Jenpen 21 September 2006
Source http://en.wikipedia.org/wiki/File:Mttscheme.png . Public domain use per Wikipedia Commons.
Colorless (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium) is reduced to purple formazan
The BIOLOG-ECO™ 96 well plates we will use contain 3 replicates of 31 carbon sources and three water control wells. Most of these substrates are commonly associated with plant root exudates and, thus, are likely to be available to your soil community. The method for community carbon source profiling that we are using is simple and rapid, but its interpretation must be carefully evaluated, recognizing that the methodology is imperfect. The following studies discuss issues and limitations of CLPP (community level physiological profiles) analysis.
These or similar references are provided in the lab Sakai site as .pdf files.
References and Resources: Biolog Carbon Source
• Garland, J.L., Mills, A.L. (1991) Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level-sole-carbon-source-utilization. Appl Environ Microbiol 57, 2351–2359.
• Garland, J.L. (1997) Analysis and interpretation of community-level physiological profiles in microbialecology. FEMS Microbiol Ecol 24, 289–300.
• Preston-Mafham, J., Boddy, l., Randerson, P.F. (2002) Analysis of microbial community functional diversity using sole carbon source utilization profiles-a critique. FEMS Microbiology Ecology. 42, 1-14.
BIOLOG Redox Dye Mix Brochure JUL07.
PROTOCOL:
Carbon source utilization patterns using BIOLOG™ Community Level Physiological Profiling (CLPP)
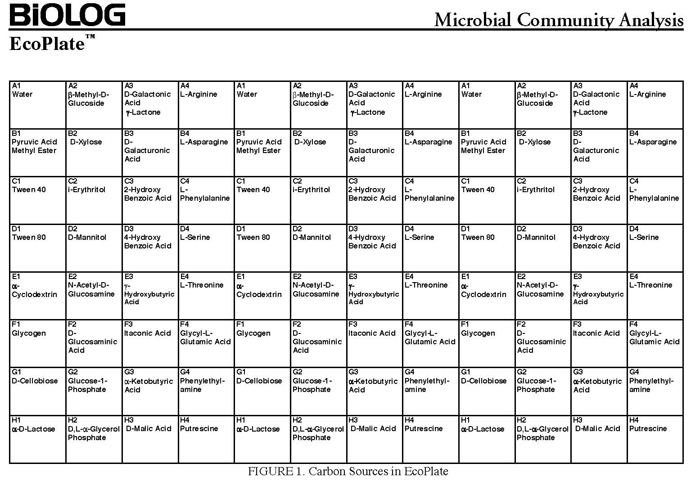
- 10mM phosphate buffer (sterile, pH 7)
- P200 and P1000 micropipets with sterile tips
- Multichannel pipet (set to deliver 100 µl) and sterile tips
- BIOLOG EcoPlate™
- sterile plastic multichannel reservoir
- Wear gloves throughout the entire protocol
- Do not cross contaminate your samples or the solutions
- Keep your work area clean: freshly disinfect your bench top before beginning
- Do not use a vortex at any point in this protocol unless it is specified that you should do so.
1. Use sterile serological pipets to make 12 mls of the appropriate dilution. Our goal is to end up with about 105 cfu in each well; therefore, you need a working solution that has 106CFUs/ml, because 0.1ml contains 105CFUs. This is an estimate that does not account for those organisms that did not grow on NA, but might grow in this community assay. You need 12 ml (10 ml for the plate and 2 ml extra) of this working solution for each BIOLOG™ECO plate in order to inoculate all the wells. You will accomplish this by pipetting 10.8 mL of 10mM phosphate buffer into a large sterile tube and adding 1.2 mL from the serial dilution that gave you 107CFUs/ ml. You can find that information in the Enumeration DATA sheet in the Data folder.
2. If you will be using a multichannel micropipet, pour this 12ml of diluted soil extract (106 cfu/ml) into a sterile reservoir (a petri dish or a v-shaped plastic reservoir). If the multichannel pipet is unavailable, forget pouring the soil extract dilution into a reservoir. You can use your P200 to dispense 100μL individual aliquots directly into each well of the BioLog plate as described in the next step.
3. Be careful to preserve the BIOLOG Eco™ plate's and its cover's sterility (eg. don't place it face down on your bench). Remove the cover and transfer 100µl of the soil extract dilution into each each well of the 96 well BIOLOG plate or use a multichannel pipet with new tips set to 100μL. If you are using the multichannel pipet, check visually the consistency of the amount of diluted extract in each of the micropipet tips after you have drawn up your aliquots to determine that you have no bubbles and that the quantity to be dispensed is the same. If the pipet tips appear unevenly filled or you have bubbles, do not dispense the inoculum into the wells! Expel the fluid back into the reservoir and start over. If you are unfamiliar with the use of multichannel pipets, ask your instructor to observe your technique.
4. Add 50μL of phosphate buffered saline (PBS) to each well (to account for dehydration over time of measurement). Note that this dilution does not change the number of cfus per well (105 cfus), but it does change the overall concentration of your soil microbes in this assay.
5. Replace the cover of the plate and label one side of the cover. DO NOT LABEL ON the top or bottom to avoid interference in the light passage during spectrophotometric readings. Use a piece of your team color tape and include your initials, lab section, date, and soil sample code.
6. Take a time 0 reading at A590nm using the SpectraMax 190 plate reader as described in the next section of these instructions.
7. After each reading, place your covered plate in a plastic container with moist paper towels and incubate it at room temp (~22C) in the location assigned for your lab section.
MEASURING MICROBIAL GROWTH
The intensity of color change is monitored in each of the wells by taking spectrophotometer readings once a day at A590 nm. You and your partners need to figure out a schedule to divide up the work of collecting these data. Make sure that you take a photo of your plate against a white background on the final day of measurement. There are a few digital cameras available or you can use your own camera.
We will use the MolecularDevices SpectraMax 190 located in the lab and the SOFTmaxPRO6.3™ program.
Time 0: Use the SpectraMax 190 spectrophotometer (with a microplate reader) found in the Microbiology lab (L302). You will measure absorbance as A590 nm. Follow the directions for setting up the instrument and software and for exporting the data to Excel spreadsheets found in the next section:
SpectraMax 190:
Located in the back of lab 302
Molecular Devices SpectraMax 190 and SoftMax Pro6.3™ software instructions

View of back of Spectra Max 190
Turn on the Spectra MAX 190 spectrophotometer using the switch located next to the plug in the back on the right hand side (as you face the spec). The drawer will open. The drawer may, or may not, close on its own. If it doesn't close, please press the DRAWER button found on the Spectra MAX control panel (pictured below). It is important to keep the drawer closed as much as possible to prevent dust from entering the spectrophotometer.


The machine will automatically start a required calibration. Allow up to 10 minutes for the Specta MAX 190 to warm up after the calibration and BEFORE you attempt to insert your plate.
The computer should be on. (If not, turn it on.)
When the warm up period is over, double click on the SOFTmaxPRO6.3 shortcut on the desktop of the computer. ![]()
The draw may open again, or you can open it manually by pushing the DRAWER button ONCE. (Drawer button is found on the Spectramax control panel)
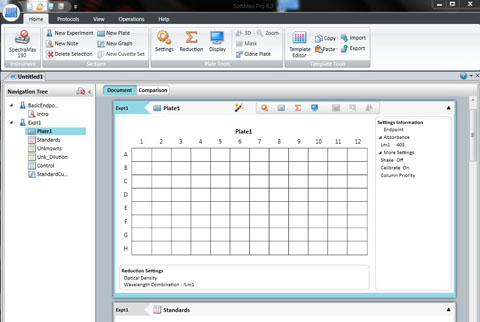
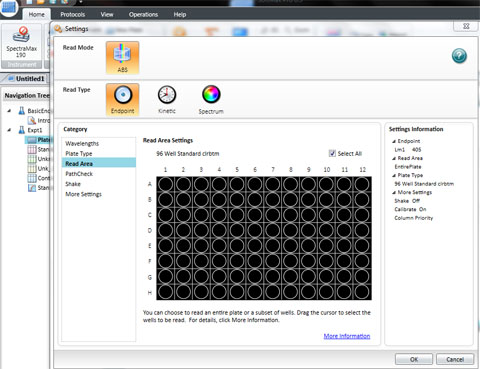
Position your 96 well plate into the tray drawer so that it fits securely in the holder and the well A1 is to the top left. Close it using the DRAWER button .
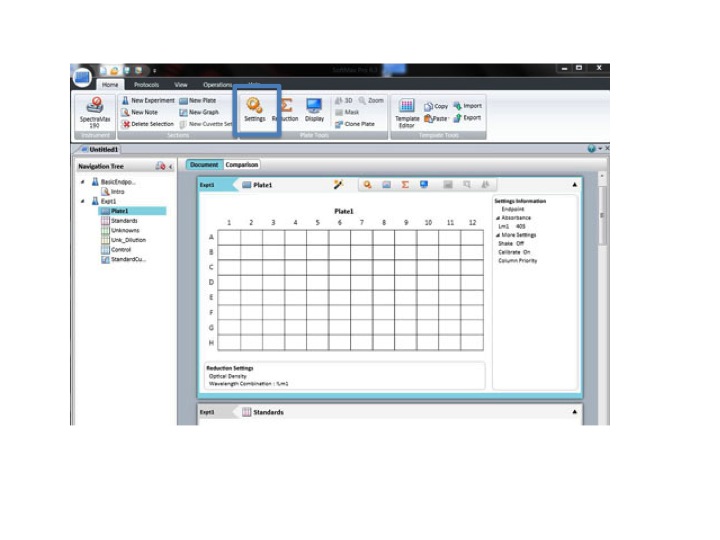
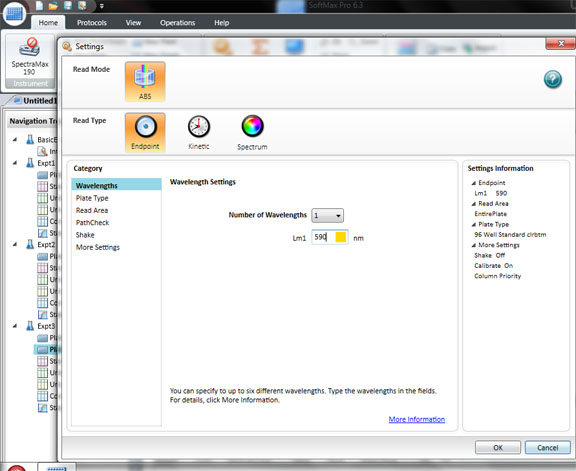
Click: SETTING
Set the wavelength to 590 for the BIOLOG Ecoplate. Check that Plate type is 96 well. Check that the Read area is the whole plate.
Click OK
The spec is ready to read all the wells in a 96 well plate. Click Read.
You will hear the spectrophotometer slit lamp passing over the plate to "read" it, meaning that it is measuring absorbance at the 590 nm wavelength best for detecting purple colored pigments. The purple color formation is a function of the total redox reactions that occurred in the well as the microbes use a carbon source metabolicaly causing the reduction of the colorless form of the dye, 5-cyano-2,3,-ditolyl tetrazolium chloride (CTC), to a purple- colored product.
The drawer will open when the readings are completed. Remove your plate and CLOSE the drawer using the DRAWER button on the face of the spectrophotometer. It is important to keep the drawer closed as much as possible to prevent dust from entering the internal parts of the machine.
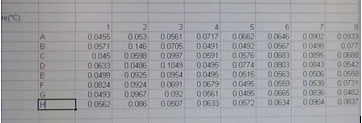
A new screen will appear with your recorded data in a 96 well template.
The data should be saved automatically in softmax pro, but please also save to our BISC209 folder on the desktop, labeling clearly with your group letter and the date.
Because the instrument computer is not networked, you will have to Save the data to a FLASH DRIVE to export it to your Sakai data file.
Export the labeled data to the Flash drive by clicking on the blue image of the plate in the upper left of the screen. From the menu that appears, click Export. Then Choose Plate1 and Output format PLATE raw (.txt). Click OK. Then SAVE AS with your site and date AND change the file type to .xls (not the default!). Save to the USB (D:) drive.
Close the Spectromax software. DON'T SAVE! (You have already saved and exported adequately.)
Shut off the SpectraMax spectrophotometer unless you know another group is waiting. DO NOT SHUT DOWN THE COMPUTER!!!
Eject the Flash drive hardware properly by right clicking on the left image on the bottom of the computer's tool bar. If that doesn't work, go to Computer--USB (D:) Eject
Remove Flash drive and take it to the lab computer facing the Spectromax and plug it into a USB port.
DATA STORAGE:
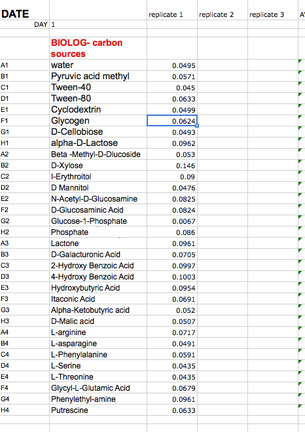
Open your team's Data folder from Sakai and download and open your group's Biolog data sheet. The file is called: BIOLOG-CMD_calc&GraphGroup__2013fa.
Then EDIT: PASTE SPECIAL: TEXT the data carefully to your team's Excel spreadsheet making SURE that you are on the appropriate day (found in tabs on the bottom of the workbook). Note that each day that you collect data has a separate page in this spreadsheet.
Copy and paste one column in the spectrophotometer 96 well format at time into your group's spreadsheet. After pasting column 1, 2 and 3 you will begin the next set of replicates (columns 4-6 then 7-9).
This is a very important step because it aligns the replicate cells properly.
When you have finished, go to Finder and eject the Flashdrive from Devices. Wait for it to disappear. Please store the flash drive in the USB port in the computer on the Spectromax. If it is not there, send a message to the class to see if someone took it home by mistake and email your instructor. You can still transfer your data to your Sakai data folder if the disk is missing by reading off the data from the Spectromax computer to your open data Spreadsheet. (The 2 computer screens are close enough to allow that.) If you need to look at the Biolog template to see if you are transferring the appropriate cells you can pull it up from the Powerpoint intro for Lab 3 found in Resources in Sakai. It is crucially important that you align the right carbon source to its value (don't forget water) and get the right well numbers transferred to the appropriate position on the Worksheet DAY (1, 2,etc) in the template workbook. Note that the Template contains a separate worksheet (tabs on the bottom) for each day within the full workbook. Make sure that you fill out the appropriate day (DAY 0, 1, 2 etc). If you miss a day, leave that page blank.
PRECAUTION: If you haven’t saved your data and another group reads their plate, your data will be overwritten and lost.
DATA ANALYSIS:
The Spreadsheet is pre-formatted with the calculations for these data. It includes the formulas to average replicate measurements each day and it will automatically subtract the background (readings in the water wells). There is a normalization for background that will be subtracted automatically (this threshold absorbance is 0.25 for each carbon source). We hope that including these pre-made calculations in the template will make your calculation of community metabolic diversity (CMD) and the data analysis of carbon source utilization patterns relatively uncomplicated. Please make sure you understand the EXCEL performed calculations. When you are sure that your data has been copied correctly into the appropriate template day, you will notice that the built in calculations will provide a final average absorbance reading for each substrate.
Fill in the last column labled: SCORE 1 for wells with OD above 0.0. Once you fill in this final column, the template will calculate the total number of positive wells out of 31 for you and the SUM will appear in a cell below your data. This is the CMD for that day. The total will also appear on the last tab of the workbook (CMDtable&graph).
Continue Isolation of Bacteria to Pure Culture as Examples of Soil Community Richness and Co-operative and Competitive Behavior
Continue to attempt to isolate to pure culture examples of your soil community membership.
Directions for Streaking for Isolation onto new solid media is found at Streaking for Isolation
Coordinate with your partners to try to isolate colonies that look different from each other. Your goal is for each student to end up with 2 pure cultures of DIFFERENT genera of bacteria from as many groups as possible.
CLEAN UP
1. All culture plates that you are finished with should be discarded in the big orange autoclave bag near the sink next to the instructor table. Ask your instructor whether or not to save stock cultures and plates with organisms that are provided.
2. Culture plates, stocks, etc. that you are not finished with should be labeled on a piece of your your team color tape. Place the labeled cultures in your lab section's designated area in the incubator, the walk-in cold room, or at room temp. in a labeled rack. If you have a stack of plates, wrap a piece of your team color tape around the whole stack.
3. Remove tape from all liquid cultures in glass tubes. Then place the glass tubes with caps in racks by the sink near the instructor's table. Do not discard the contents of the tubes.
4. Glass slides or disposable glass tubes can be discarded in the glass disposal box.
5. Make sure all contaminated, plastic, disposable, serologic pipets and used contaminated micropipet tips are in the small orange autoclave bag sitting in the plastic container on your bench.
6. If you used the microscope, clean the lenses of the microscope with lens paper, being very careful NOT to get oil residue on any of the objectives other than the oil immersion 100x objective. Move the lowest power objective into the locked viewing position, turn off the light source, wind the power cord, and cover the microscope with its dust cover before replacing the microscope in the cabinet.
7. If you used it, rinse your staining tray and leave it upside down on paper towels next to your sink.
8. Turn off the gas and remove the tube from the nozzle. Place your bunsen burner and tube in your large drawer.
9. Place all your equipment (loop, striker, sharpie, etc) including your microfuge rack, your micropipets and your micropipet tips in your small or large drawer.
10. Move your notebook and lab manual so that you can disinfect your bench thoroughly.
11. Take off your lab coat and store it in the blue cabinet with your microscope.
12. Wash your hands.
Assignment
Graded Assignment:
Assignment (20 points): Explain The Great Plate Count Anomaly. Instructions: The Great Plate Count Anomaly.
To Do Before the Next Lab:
The timely submission of daily BIOLOG-workbook entries to the DATA folder for your worksite is expected.