Micellar Electrokinetic Capillary Chromatography - Robin Levinson
Introduction and Background
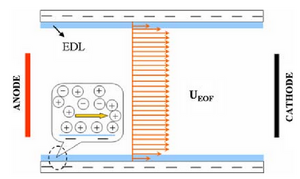
Electrophoresis relies on electrosmotic flow (EOF), which is when a polar liquid induces charge on a stationary surface. Then, an applied potential drives liquid motion between the charged ends. Positively charged molecules are driven towards the negatively charged cathode, dragging the bulk solution. Additionally, with the wall of the capillary negatively charged, the positively charged molecules accumulate near the wall.
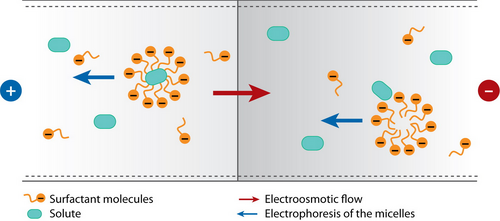
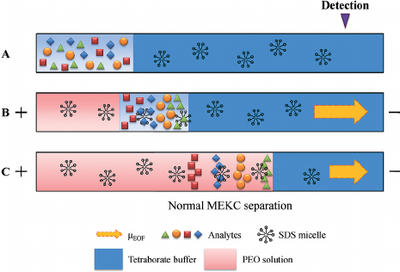
Free-zone electrophoresis done in a capillary is referred to as capillary zone electrophoresis (CZE) (see Capillary Zone Electrophoresis - Andrew Maloney). In CZE, the capillary is negatively charged, so analytes migrate toward the cathode due to strong EOF. The migration velocity is based primarily on the ratio of charge to molecular size [1]. Since separation by CZE relies on the charge of the analyte, it is unable to separate neutral analytes. As a result, in 1984, micellar electrokinetic capillary chromatography (MEKC) was developed to separate neutral compounds [2]. This separation is achieved by the addition of ionic micelles to the electrophoretic medium. A micelle is a self-assembled, single layer particle formed from amphiphilic molecules. A portion of the neutral analyte is incorporated into the micelle and can therefore be separated as it migrates by electrophoresis with a velocity different than that of the bulk solution which is driven by EOF [1]. The EOF velocity (veo) opposes the electrophoretic velocity of the micelle (vep(mc)) at a greater magnitude, resulting in two distinct phases known as the stationary phase and the mobile phase [2]. The existence of these two phases gives this process the classification of chromatography; the micelle acts as the stationary phase in chromatography and is here referred to as the pseudostationary phase (PSP). The surrounding solution acts as mobile phase [1]. The micellar solution is prepared from ionic surfactant dissolved in a buffer at a concentration higher than the critical micelle concentration (CMC), which is the free surfactant concentration in equilibrium with the micelle. The CMC can be measured by electrical conductivity, surface tension, light scattering, spectrophotometry, among several other techniques. For sodium dodecyl sulfate (SDS), the most common surfactant used in electrophoresis, the CMC is about 8 mM in pure water, but varies based on its buffer and temperature. Detection of analytes is generally done by photometric methods, laser-induced fluorescence, mass spectrometry, or electrochemical methods [1].
Theory
There are several equations that describe the process of MEKC [1]. The first one is the retention factor, k, which can be defined as
where nmc is the amount of analyte incorporated into the micelle, and naq is amount free of micelle or amount in the aqueous phase. Using the retention factor, one can calculate the migration time of the analyte:
where t0 is the retention time of the solute which is not solubilized by the micelles (EOF marker), tmc is the retention time of the solute which is completely solubilized by the micelles (micelle marker). Based on this equation, it can be seen that the migration time of the neutral analyte is bound between t0 (when k=0) and tmc (when k=∞). When there is no EOF, the retention time becomes
In this case, the micelle migrates through the aqueous phase and acts as the mobile phase, despite it corresponding to the stationary phase in conventional chromatography. The net migration velocity of the micelle can also be calculated by using the EOF/bulk flow velocity (veo) and the electrophoretic velocity of the micelle (vep(mc)):
The added velocities are vectors of opposite signs. The migration velocity is therefore also a vector quantity, and is considered positive when the migration is towards the cathode. Hence, the EOF velocity is generally greater than the electrophoretic velocity.
Advantages and Disadvantages
Advantages
- limited elution time means relatively short separation time [2]
- useful for biological samples [2]
- separates ionic and neutral compounds at high efficiencies [4]
- high separation power [4]
- only requires a few nanoliters of sample [4]
- can separate small molecules
- cheaper than HPLC
- can separate chiral compounds [7]
Disadvantages
- limited elution time limits peak capacity of technique [2]
- generally limited to compounds which are reasonably soluble in the mobile phase [2]
- low sensitivity in low concentrations [6]
Applications
Food analysis
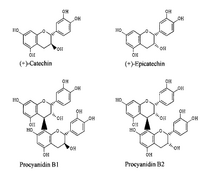
Procyanidins and their monomers are compounds found in several plant species and are often found in fruit peels and in the seed coat of legumes. These compounds are comprised of phenolic oligomers primarily composed of (+)-catechin and (-)-epicatechin groups. Certain procyanidins have shown anti-HIV, anti-ulcer, and anti-allergy activity, which has drawn a lot of attention to this class of molecules. Procyanidin supplements have also been positively associated with antioxidant activity in rats. MEKC has proven to be the superior method of analysis of procyanidins after using high-performance liquid chromatography (HPLC), thin layer chromatography (TLC), and nuclear magnetic resonance (NMR) spectroscopy. Spectroscopic methods require sample pre-treatments to concentrate the sample or make it optically transparent, and HPLC takes longer than thirty minutes. MEKC was able to separate seven compounds in less than five minutes [4].
Environmental analysis
Most sewage treatment plants are unable to completely remove hydrophilic and stable compounds, so the compounds end up remaining in bodies of water. One such class of compounds are nonsteroidal anti-inflammatory drugs (NSAIDs). MEKC is one method that can be used to identify these anti-inflammatory drugs in water. Because MEKC is not very sensitive at low concentrations, preconcentration procedures are necessary before the separation can take place. Two main preconcentration methods include sample stacking and sweeping. Sample stacking involves the sudden change in the electrophoretic migrating velocities of the sample solution and background analyte due to the change of magnitude of the electric field. Sweeping is based on the injection of a large amount of sample without the stationary phase and the accumulation of analytes which penetrates the sample. These techniques can increase the concentration sensitivity by a hundred times its original concentration [7].
Pharmaceuticals
Micellar electrokinetic chromatography is used the pharmaceutical industry for enantiomer separation, separation of amino acids and closely related peptides, separation of very complex mixtures, determination of drugs in biological samples, and separation of electrically neutral drugs [7]. Enantiomer separation can be achieved when a chiral surfactant is used for the mobile phase. Drug standards require 0.1% of impurities to be identified, and this can be done using MEKC after using a preconcentration procedure such as sample stacking. Peaks generated on retention time graphs are useful in determining drug purity, similar to HPLC. Changing the micellar solution or using additives can change the selectivity for the separation of drugs. This process has allowed more than eighteen amino acid derivatives to be separated in thirty minutes. Using MEKC over CZE for the separation of peptides is advantageous because there is much more freedom for selectivity manipulation.
References
- Terabe, S. Capillary Separation: Micellar Electrokinetic Chromatography. Annual Review of Analytical Chemistry 2009, 2: 99-120. https://doi.org/10.1146/annurev.anchem.1.031207.113005
- Sepaniak, M.J.; Burton, D.E.; Maskarinec, M.P. Micellar Electrokinetic Capillary Chromatography. Ordered Media in Chemical Separations 1987, 342: 142-151. https://doi.org/10.1021/bk-1987-0342.ch006.
- Terabe, S. Micellar Electrokinetic Chromatography. Analytical Chemistry 2004, 76(13): 240A-246A. https://doi.org/10.1021/ac0415859.
- Cifuentes, A.; Bartolomé, B.; Gómez-Cordovés, C. Fast determination of procyanidins and other phenolic compounds in food samples by micellar electrokinetic chromatography using acidic buffers. Electrophoresis 2001, 22: 1561-1567. https://doi.org/10.1002/1522-2683(200105)22:8<1561::AID-ELPS1561>3.0.CO;2-P
- Kang, Y.; Li, D. Electrokinetic Motion of Particles and Cells in Microchannels. Microfluid Nanofluid 2009, 6: 431-460. https://doi.org/10.1007/s10404-009-0408-7.
- Maijó, I.; Borrull, F.; Aguilar, C.; Calull, M. Determination of Anti-Inflammatory Drugs in River Water by Sweeping-Micellar Electrokinetic Capillary Chromatography. Journal of Liquid Chromatography and Related Technologies 2012, 35: 2134-2147. https://doi.org/10.1080/10826076.2011.629386.
- Nishi, H. Pharmaceutical applications of micelles in chromatography and electrophoresis. Journal of Chromatography A 1997, 780(2): 243-264. https://doi.org/10.1016/S0021-9673(97)00347-6.
- Tsai, I.C.; Su, C.; Hu, C.C.; Chiu, T. Simultaneous determination of whitening agents and parabens in cosmetic products by capillary electrophoresis with on-line sweeping enhancement. Analytical Methods 2014, 6(19). https://doi.org/10.1039/C4AY00985A.
