Capillary Zone Electrophoresis - Andrew Maloney
Background
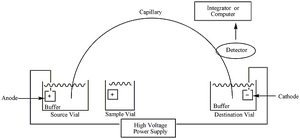
The basic principle of electrophoresis is the movement of charged particles as the result of an electric gradient. Capillary electrophoresis frequently substitutes the gel that is typical of an electrophoresis medium, with a capillary. These tubes typically have dimensions of roughly 50 μm by 100cm in length, and are operated from 20-30kV[1]. These high voltages enable a fast separation, and well-defined “zones” of analyte. Each end of the capillary tube is dipped into a buffer solution and one end is put in the inlet solution with the anode and the other with the end runs through the detector and to the cathode vial. The analytes will migrate through the capillary due to the electric gradient and will separate into bands from each other based on size. In zone electrophoresis these zones are well defined with areas of buffer separating zones. As the zones pass the detector they are typically detected with UV absorption near the 200nm range. Because of the high voltage, and thus tighter bands, the resultant electropherogram will display narrow peaks that correspond to the respective analyte. Unlike in capillary isotachophoresis the capillary zone electrophoresis should separate into bands with buffer between bands [2]. These buffer bands mean that the electropherogram should return to 0 between analyte detections.
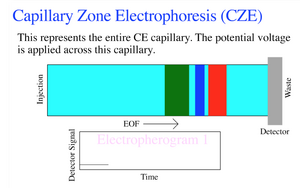
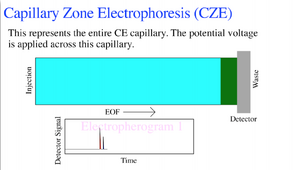
Governing Equations
Some of the governing equations for the flow regime in capillary zone electrophoresis include:
The velocity of an analyte v:
v=μE = μV/L
Where μ is the electrophoretic mobility, V is the voltage, and L is the length of the tube.
Therefore the time for a zone of analyte to transverse the tube t is governed by:
t=L/v or L*L/μV
The molecular diffusion σ that contributes to the broadening of the zones can be modeled by:
σ=2Dt
Where D is the diameter of the capillary and combined with the previous equation, the spatial variance can be modeled by:
σ=2DL*L/μV
[3]
A relevant equation for analyzing electropherograms is the resolution which is the distance between peaks divided by the average peak width [7].
Transition to the Microscale
In the case of Capillary Electrophoresis, there are numerous benefits to transitioning to the microscale including the ability to apply high voltages because of the heat transfer on the small diameter of the capillary [6]. This high voltage is what yields the strong zone definition and allows the process to be run relatively quickly. The small diameter allows for heat to be generated uniformly throughout the tube's cross section and dissipated to the tube walls [1].
Factors to consider at the microscale are viscosity, pH, and polarity of the analytes. All three of these factors affect the flow within the capillary. Viscosity and pH can form gradients which can cause zone dispersion and vary the time that the analytes spend near the center or the wall of the capillary. Polarity of the anayltes can cause sample overloading, where a sufficiently concentrated substance can perturb the chemical and physical properties of the medium. As most analytes have acidic or basic properties, a high concentration may decrease the mobility of the substance or affect the conductive properties of the medium. Therefore, a low concentration of analytes to electrolyte buffer is used in Capillary Zone Electrophoresis to avoid sample overloading. The lower concentration and the smaller volume also place a higher priority on greater levels of detection sensitivity [1].
Methods of Detection
There are two primary methods for detection of analytes in Capillary Zone Electrophoresis, fluorescence and UV absorption. Both of these methods are "on-column" techniques where an incident light beam is shined onto the capillary [1]. Both of techniques are highly sensitive, however, fluorescence detection does not work well for protein's unless it has a specific marker such as FITC [1] [4]. Proteins are usually not good candidates for fluorescence detection because they have high variability and weak fluorescence [1].
Applications
One of the primary applications for capillary zone electrophoresis is in the separation and/or detection of biomaterials on a microscale. More specifically, capillary zone electrophoresis offers a highly-efficient method for amino acid separation [4]. The narrow peaks provided because of the high voltage make it a strong separation method for mixtures of amino acids.
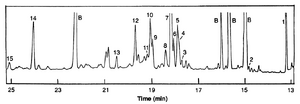
Capillary zone electrophoresis can also be applied to separate larger things like proteins. For a proper run of a capillary zone electrophoresis separation with protiens, the proper pH level needs to maintained via the buffer so as to overcome the Coulombic repulsion of the protein and the silica walls. [5]. This technique also offers a low cost and user friendly way to analyze the quality of antibiotics such as penicillin. Paul et al. describe a method for analyzing the quality of pre or post commercialized antibiotics using capillary zone electrophoresis [6].
References
1. James W. Jorgenson. Capillary Zone Electrophoresis New Directions in Electrophoretic Methods. March 18, 1987 , 182-198. DOI: http://dx.doi.org/10.1021/bk-1987-0335.ch013
2. Chasteen, T. G. “Modes of Capillary Electrophoresis.” www.shsu.edu/~chm_tgc/primers/pdf/CEs.pdf.
3. Jorgenson, J. W., & Lukacs, K. D. (1983). Capillary zone electrophoresis. Science, 222(4621), 266-272.
4. Y.-F. Cheng and N. J. Dovichi, "Subattomole amino acid analysis by capillary zone electrophoresis and laser-induced fluorescence". Science 28 Oct 1988: Vol. 242, Issue 4878, pp. 562-564 DOI: http://dx.doi.org/10.1126/science.3140381
5. Lauer, . H.; McManlgHI, D. Anal. Chem. 1986, 58, 166-170 DOI: 10.1021/ac00292a041
6. Jorgenson, James W., and Krynn DeArman Lukacs. "Capillary zone electrophoresis." Science, vol. 222, 1983, p. 266+. Academic OneFile, Accessed 23 Feb. 2018.
7. Paul, P.; Sänger-van de Griend, C.; Adams, E.; Van Schepdael, A. ELECTROPHORESIS 2018. Wiley. DOI: 10.1002/elps.201800033, accessed 12 April, 2018.
8. Perry, S. ChE590E Microfluidics and Analysis. Lecture 6: Separations. 2018. University of Massachusetts, Department of Chemical Engineering.
9. By Apblum [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/)], via Wikimedia Commons