IGEM:Harvard/2006/Cyanobacteria/Notebook/2006-8-5
<html><style type='text/css'> .tabs {
font-size:80%;
font-weight:none;
width: 100%;
color: #FFFFFF;
background:#FFFFFF url("/images/5/54/DarkgreenTab-bg.gif") repeat-x bottom;
}
.tabs li {
background:url("/images/3/36/DarkgeenTab-left.gif") no-repeat left top;
}
.tabs a,.tabs strong {
background:url("/images/d/d3/DarkgreenTab-right.gif") no-repeat right top;
color:#FFFFFF;
padding: 3px 10px 3px 4px;
}
.tabs strong{
color:#CCFF00;
background-image:url("/images/b/b1/DarkgreenTab-right_on.gif");
}
.tabs a:hover{
color:#66FF00;
}
</style></html>
To-do
Inoculate transformants of KaiA + bkb and KaiB + bkb ligations(complete)Inoculate Top10F' transformants (J04450 plasmid)(we don't need to use it for anything else; it was only a test of lacIq)Run the X-P digest of J04450 on a gel(the gel lacked a ladder - look below)Extract and purify the J04450 insert if it exists
Miniprep the J04450 in Top10 culture that we inoculated last night(complete)- (Maybe) Perform another genomic PCR extraction of KaiA/B and KaiC
Innoculate transformants
We had one colony on each plate of KaiA+bkb and KaiB+bkb high copy. Innoculated in 8mL of LB + 8uL of amp stock; growing currently in 37 shaker. Plates put in -4 by gel imaging.
Top10F' transformants are constitutively red
It looks like the Top10F' cells we transformed with J04450 are constitutively expressing RFP, despite the lacIq gene on the Top10F' genome.
Fusion Proteins
Miniprep 12 of Perry's cultures
Gel image of J04450 + bkb X-P digest from yesterday
Looks like there was a mistake with the ladder or our ladder is broken.
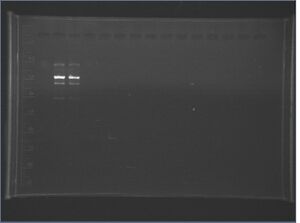
E-S digest of low-copy plasmid and X-P digest of J04450 + bkb
We performed an E-S digest of the low-copy plasmid that we miniprepped yesterday, and (another) X-P digest of J04450 + bkb so we can get the J04450 insert. We plan to ligate the J04450 insert with the low-copy vector to test the leakiness of the Lac promoter with the low-copy vector.
Each digest had the following mix:
- 19.25 uL DNA (low-copy plasmid or J04450 + bkb)
- 2 uL H2O
- 2.5 uL buffer (EcoRI buffer or Buffer 3)
- 0.5 uL enzyme 1 (EcoRI or XbaI)
- 0.5 uL enzyme 2 (SpeI or PstI)
- 0.25 uL BSA
25 uL total volume.
Ligation of KaiA/B + bkb and transformation
Religated KaiA and B into the RFP bkb backbone, this time using 50uL of cells for each of KaiA, B and 25 uL for the negative and positive control, and using all of the ligation product. more colonies resulted.