IGEM:Harvard/2006/Cyanobacteria/Notebook/2006-8-11
<html><style type='text/css'> .tabs {
font-size:80%;
font-weight:none;
width: 100%;
color: #FFFFFF;
background:#FFFFFF url("/images/5/54/DarkgreenTab-bg.gif") repeat-x bottom;
}
.tabs li {
background:url("/images/3/36/DarkgeenTab-left.gif") no-repeat left top;
}
.tabs a,.tabs strong {
background:url("/images/d/d3/DarkgreenTab-right.gif") no-repeat right top;
color:#FFFFFF;
padding: 3px 10px 3px 4px;
}
.tabs strong{
color:#CCFF00;
background-image:url("/images/b/b1/DarkgreenTab-right_on.gif");
}
.tabs a:hover{
color:#66FF00;
}
</style></html>
Ligation assay
We want to keep our ligation assay modest in size because each ligation requires at least a transformation (and possibly an inoculation and miniprep as well).
| Ligation # | Insert:Vector | Ligation time |
| 1 | 1:1 | 5 min |
| 2 | 3:1 | 5 min |
| 3 | 6:1 | 5 min |
| 4 | 1:1 | 15 min |
| 5 | 3:1 | 15 min |
| 6 | 6:1 | 15 min |
| 7 | 1:1 | 30 min |
| 8 | 3:1 | 30 min |
| 9 | 6:1 | 30 min |
ToDo
Digestion of GeneArt plasmids with X-P so they can be ligated immediately into J04500 (Lac + RBS)(Dave)Transformation/Plating of GeneArt plasmids(Hetmann)Running digestion assay products + J04500 on a gel(Hetmann)Gel-purify a lots of things (GFP dev\X-P, pSB4A3\S-P, J04500\S-P)~~---- Ligation assay (GFP dev\X-P + pSB4A3\X-P) along with J04500\S-P + KaiA\X-P, transformation
- Prepare KaiA for sequencing
Gel-purify undigested KaiA + RFP bkb(H.C.) (Jeff) (Mistake made, explained below)- Prepare sequencing order with BB primers (Jeff)
Perry's Pet Projects for Personal Persual (Peng)- Frozen stocks of all his stuff & innoculation
Colony PCRPost-PCR gel (egel)
Digestion of GeneART plasmids
For each kaiA, kaiB, and kaiC plasmid:
- 4 uL DNA (~200 ng/uL gives 800ug DNA)
- 17.25 uL H2O
- 2.5 uL buffer (Buffer 3)
- 0.5 uL enzyme 1 (XbaI)
- 0.5 uL enzyme 2 (PstI)
- 0.25 uL BSA
25 uL total volume.
MM:
- 4x17.25= 69 dh20
- 4x2.5= 10 buffer 3
- 4x0.5 = 2 XbaI
- 4x0.5 = 2 PstI
- 4x0.25 = 1 BSA (be sure to mix well!)
pipet 21 into each tube containing the DNA
Digested at 37°C for 6 hrs, heat shock at 80°C for 20 min, hold at 4°C.
Tubes labeled A,B,C respectively, with 8/11 on the side.
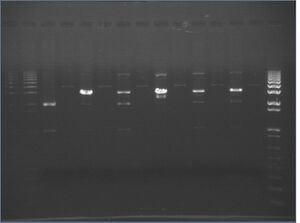
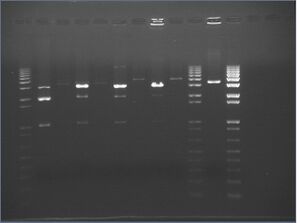
Digest Gels
Two 1% agarose gels were made:
Click for legend

Click for legend
Click for legend

Click for legend
- R0010+E0241 is in psB1A2 (2079bp)
- R0010+E0241 \ X-P is 200bp+795=995bp+6(mixed)+16+6(sides)=~1023kb
- The X-P cut should give two fragments of ~2.1kb and 1023bp; together, the two segments should be around 3 kb to 3.1 kb
- The plasmid pSB4A3 is 3339bp (\S-P cut makes the plasmid linear)
- pSB4K3+J04500 is about 3409bp (pSB4K3 is a low-copy plasmid)
Results:
It looks as if:
- The digest depends on the amount of enzyme used
- 0.5 uL of enzyme gave brighter bands than 1 uL of enzyme
- The digest might be dependant on the PCR machine used
- The best digests for R0010, in order w/regards to the insert (1kb, is):
- 0.5 uL enzyme for 12 hours
- 1.0uL enzyme for 12 hours
- 0.5uL enzyme for 2 hours
- 0.5uL enzyme for 16 hours
- The 2 hour water bath appears to be better than 4 or 6 hours in the PCR. However, the water bath wasn't exactly 37C-- it was closer to 40C most of the time.
- These results are a bit inconclusive; either 40C in a water bath over a PCR is better, or the digest is just finniky.
What this tells us:
- Always use 0.5uL ea. enzyme for double digest
- Use 12 hour digests if you have time; otherwise, a 2 hour water bath seems to work just as well (perhaps around 40°C).
Gel purification of undigested KaiA + bkb #4
I extracted and purified the undigested KaiA + bkb #4 from 2006-8-10. We intended to send this sample off for sequencing, since we're using an \X-P digest of this same strain for our ligation into J04500.
However, I made a mistake in the gel purification by eluting straight into the spin column instead of a clean tube. The DNA is probably unsalvageable since it's mixed up with Buffer QG and Buffer PE (though I heated it at 60C for 10 min to evaporate whatever ethanol remained).
Gel Purification of digest
Gel purified ten samples from the above digest; including the psB4A3 backbone \SP, psB1A2 backbone \XP, R0010+E0241 \XP, psB4A3 backbone noncut (we think?), and J04500 \SP. These are in the "Digest Assay Products" box. Note that we are not sure about the psB4A3 contents becuase of the gel; it looks like it runs ~4kb, which is bigger than expected - do not use these products for ligations. NOT CIP-PED
This DNA was eluted in 30 uL H2O.
Mouse-anti-GFP antibodies
In refrigerator in our isle.
Redigest of pSB4A3\S-P, and pSB4A3\X-P
The pSB4A3 (low-copy plasmid) didn't digest quite right. It appears to be 4kb long instead of the expected 3.3kb. We will redigest it, along with an X-P digestion to look for an insert. This time we'll also run it alongside undigested plasmid for comparison.
We used ~10 uL plasmid instead our usual ~20 uL because we ran out of pSB4A3.
pSB4A3\S-P
- 12 uL pSB4A3
- 9.25 uL H2O
- 2.5 uL Buffer 2
- 0.5 uL SpeI
- 0.5 uL PstI
- 0.25 uL BSA
pSB4A3\X-P
- 10 uL pSB4A3
- 11.25 uL H2O
- 2.5 uL Buffer 3
- 0.5 uL XbaI
- 0.5 uL PstI
- 0.25 uL BSA
pSB4A3 (undigested)
- 10 uL pSB4A3
- 12.25 uL H2O
- 2.5 uL Buffer 2
- 0.25 uL BSA
Transformation
A transformation of KaiA, KaiB, and KaiC was completed in 25 uL of Top10 competent cells, with a negative control (ddH2O). A total of 3 uL, each, was used.
Additional note:
- The incubation of KaiA, KaiB, and KaiC in the competent cells on ice lasted more than the recommended 30 minutes. According to Lewis, the incubation lasted for 10 minutes longer than planned.