IGEM:Harvard/2006/Cyanobacteria/Notebook/2006-7-20
From OpenWetWare
Jump to navigationJump to search
<html><style type='text/css'> .tabs {
font-size:80%;
font-weight:none;
width: 100%;
color: #FFFFFF;
background:#FFFFFF url("/images/5/54/DarkgreenTab-bg.gif") repeat-x bottom;
}
.tabs li {
background:url("/images/3/36/DarkgeenTab-left.gif") no-repeat left top;
}
.tabs a,.tabs strong {
background:url("/images/d/d3/DarkgreenTab-right.gif") no-repeat right top;
color:#FFFFFF;
padding: 3px 10px 3px 4px;
}
.tabs strong{
color:#CCFF00;
background-image:url("/images/b/b1/DarkgreenTab-right_on.gif");
}
.tabs a:hover{
color:#66FF00;
}
</style></html>
To-do
- Reinoculate the Hetmann Six cultures
- Run and image gels of the colony PCR we did last night
- Send CY013 and CY014 to Genewiz again
- Transform TP (the original Topo + KaiABC plasmid) to grow up more
Gel Images
- The following gel images are from the same PCR reaction that was completed last night.
- The two gel images below are of the same gel; there was a bubble between the gel and the plate, and the gel was lifted in order to remove the bubble.
- The following gel images are from the same PCR reaction that was completed last night.

Click for legend

Click for legend
- The following gel images are of the same PCR reaction as the preceding gel; this gel was run because of two things:
- As is seen, the bubble caused the gel to run incorrectly. Note: the bubble formed probably due to some problem with the gel electrophoresis machine; Perry had problems running the gel, and both his gel and our gel were superheated after they had finished running.
- There were many air bubbles in the gel, and this had been a regular problem in the last week. After being supervised by Nick, we re-made the gels so that they contained none of these air pockets.
- The following gel images are of the same PCR reaction as the preceding gel; this gel was run because of two things:
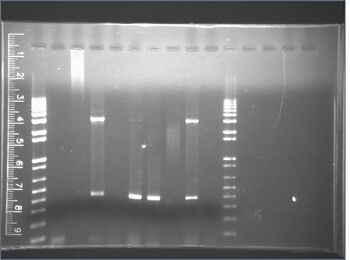
Click for legend
Nick's tips for gels
- When microwaving, be certain that the broth does not boil for too long, because this will evaporate the water and the % of agarose will be higher for the gel
- Take the flask out when this happens and gently swirl
- Make sure that the comb and the trays are clean
- Let the flask cool to a temperature comfortable to hold in your palm
- Pour gently to avoid bubbles
- When there are bubbles in the gel, use the larger end of a pipet tip to scoop the bubble out
- Cover the gel with foil to avoid bugs
- When microwaving, be certain that the broth does not boil for too long, because this will evaporate the water and the % of agarose will be higher for the gel
Retransformation of our first Topo + KaiABC plasmid
I (Jeff) retransformed our first miniprepped Topo + KaiABC plasmid into TOP10 competent cells, since we didn't have enough plasmid to use for sequencing (Genwqiz didn't receive our order modification in time).
Since most of the miniprepped DNA was already succesfully ligated with Topo, I mixed a very small amount (2 µL of 50x dilution) with 50 µL of competent cells. I also mixed 10 µL competent cells with 2 µL H2O for a negative control. I spread 5 plates overnight: 4 experimental and 1 negative control.