Bryan Hernandez/20.109/Lab notebook/Module 1/Day 6
Introduction
Antibodies are useful tools in the lab. Today you will use antibodies to detect a protein on a blot. This technique, called Western analysis, can give you information about the size and concentration of the protein in the pool that was separated by SDS-PAGE. In your case today, you will use a Western to identify the expression of an M13 protein in infected cells and to determine if phage are present in the supernatant of infected cells. In general, detection depends on which antibody you choose, and the quality of your results depends largely on the quality of that antibody.
For Western analysis, a high quality antibody can have a relatively low affinity for its target protein. This is because the target is localized and concentrated on a blot, allowing the antibody to bind using both antibody “arms” thereby strengthening the association. Even an antibody that is loosely bound to the blot under these circumstances may dissociate then re-associate quickly since the local concentration of the target protein is high. The lower limit for protein detection is approximately 1 ng/lane, a value that varies with the size of the protein to be detected and the Western blotting apparatus that is used. For most acrylamide gels, the protein capacity for each lane is usually 100 to 200 ug (that would be 20 ul of a 5-10 ug/ul protein preparation). Thus 1 ng represents a protein that is approximately 0.001-0.002% of the total cellular protein (1 ng out of 100,000-200,000 ng). Obviously proteins that make up a more significant fraction of the total protein population will be easier to detect.
Many species can be used to raise antibodies. Most commonly mice, rabbits, and goats are immunized, but other animals like sheep, chickens, rats and even humans can be used. The protein used to raise an antibody is called the antigen and the portion of the antigen that is recognized by an antibody is called the epitope. Each antibody can recognize only a small portion of its antigen, typically 5 to 6 amino acids. Some antibodies are monoclonal, or more appropriately “monospecific,” and recognize one epitope, while other antibodies, called polyclonal antibodies, are in fact antibody pools that recognize multiple epitopes. We will be using a monoclonal antibody against the myc-epitope today, but for the sake of completion, the origin of both polyclonal and monoclonal antibodies are described.
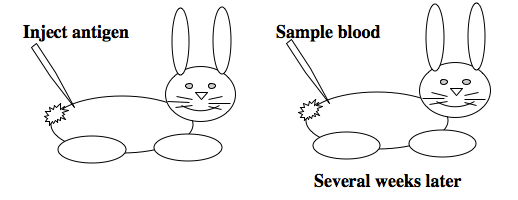
To raise polyclonal antibodies, the antigen of interest is first purified and then injected into an animal. To elicit and enhance the animal’s immunogenic response, the antigen is often injected multiple times over several weeks in the presence of an immune-boosting compound called adjuvant. After some time, usually 4 to 8 weeks, samples of the animal’s blood are collected and the cellular fraction is removed by centrifugation. What is left, called the serum, can then be tested in the lab for the presence of specific antibodies. Even the very best antisera have no more than 10% of their antibodies directed against a particular antigen. The quality of any antiserum is judged by its purity (that it has few other antibodies), its specificity (that it recognizes the antigen and not other spurious proteins) and its concentration (sometimes called its titer). Animals with strong responses to an antigen can be boosted with the antigen and then bled many times, so large volumes of antisera can be produced. However animals have limited life-spans and even the largest volumes of antiserum will eventually run out, requiring a new animal for immunization. The purity, specificity and titer of the new antiserum will likely differ from that of the first batch. High titer antisera against bacterial and viral proteins can be particularly precious since these antibodies are difficult to raise; most animals have seen these immunogens before and therefore don’t mount a major immune response when immunized. Antibodies against toxic proteins are also challenging to produce if they make the animals sick.
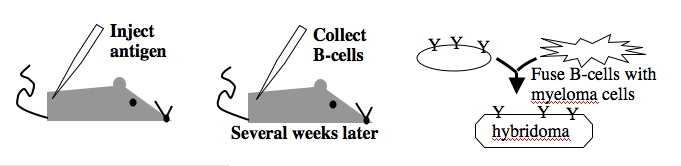
Monoclonal antibodies overcome many limitations of polyclonal pools in that they are specific to a particular epitope and can be produced in unlimited quantities. However, more time is required to establish these antibody-producing cells, called hybridomas, and it is a more expensive endeavor. Antibody-secreting cells are first isolated from an immunized animal, usually a mouse, and then fused with an immortalized cell line such as a myeloma. The fusion can be accomplished by incubating the cells with polyethylene glycol (antifreeze), which facilitates the joining of the plasma membranes of the two cell types. A fused cell with two nuclei can be resolved into a stable hybridoma after mitosis. The unfused antibody-secreting cells have a limited lifespan and so die out of the hybridoma population, but the myelomas must be removed with some selection against the unfused cells. Production of stable hybridomas is tedious and difficult but often worth the effort since monoclonal antibodies can recognize covalently-modified epitopes specifically. These are invaluable for experimentally distinguishing the phosphorylated or glycosylated forms of an antigen from the unmodified forms.
Making antibodies is big business since they can be useful therapeutics. The 2002 market for monoclonal therapeutic antibodies was estimated at almost $300 million and total therapeutic antibody market was estimated at more than $5 billion. These markets are expected to grow considerably, although successful antibody treatments may require clever engineering discoveries to “humanize” antibodies raised in other animals, as well as speedier development, well-protected patents, improvements in drug-delivery methods and cost efficient production of the therapeutics.
Protocols
Part 1: Probe Western blot
- You should retreive the blot that you made last time and pour the TBS-T + milk solution into a 50 ml conical tube.
- Wear gloves and cut the blot next to the markers in the middle of the blot.
- Place the blot lanes 1-4 in one blotting container, and the other portion of the blot (lanes 5-10) in another container.
- Add 15 ml of TBS-T + milk to each.
- Add 30 ul of anti-myc antibody to the container with lanes 1-4.
- Add 30 ul of anti-p8 or anti-p3 antibody to the container with lanes 5-10.
- Cover the containers, label with your team color and the antibody they contain, and place on the platform shaker for 45 minutes. During this time, a representative from MIT's Environment, Health and Safety Office will speak with us about biosafety as it relates to cell culture work.
- Pour the antibody solutions into conical tubes, writing the identity of the antibody and the date on the tube.
- Give the blots a quick rinse with TBS-T, enough to cover the blot (volume is not critical here).
- Wash the blot on the platform shaker 2 times with TBS-T at room temperature, five minutes per wash. Again the volume of the wash solution is not critical.
- Add secondary antibody (1:1000 Goat-antimouse-alkaline phosphotase) in TBS-T and incubate on the platform shaker at room temperature for 30 minutes.
- Wash the blot as before.
- Add developing solution and shake on the platform shaker watching for color to develop. Rinse the blot with water when bands are evident (you should anticipate what size protein you are looking for) but before the background of the blot becomes discolored. One of the teaching faculty will scan the blot and post the results for you.
Part 2: M13.1
As a class we will consider the design of M13.1 and refine the sketch that was initially suggested. The synthesis order will only be placed if we feel we have a reasonable chance of making a successful (i.e. infective) phage.
DONE!
For next time
- Prepare for the start of Module 2 by browsing through 20.109:Module 2, looking in detail at the introduction for 20.109(S07): Start-up signal measurement
- Read about calmodulin ("CaM") at the protein data bank (try searching for "molecule of the month calmodulin" for a nice summary). You should also familiarlize yourself with the structure of calmodulin by reading the original description in the 1998 paper by Babu, Bugg, and Cook: [1]
- Familiarize yourself with cell culture work by reading 20.109(S07):Guidelines for working in the tissue culture facility
Reagents list
- TBS-T Tris-Buffered Saline + Tween
- monoclonal anti-myc (9E10), raised in mouse cells
- monoclonal anti-p3 from NEB, raised in mouse cells
- monoclonal anti-p8 from GE-Amersham, raised in mouse cells
- polyclonal antimouse-AP from BioRad, raised in goat
- BioRad AP detection reagents
- 1 ml 25x detection stock + 24 ml H2O with 0.25 ml solnA and 0.25 ml solnB.
