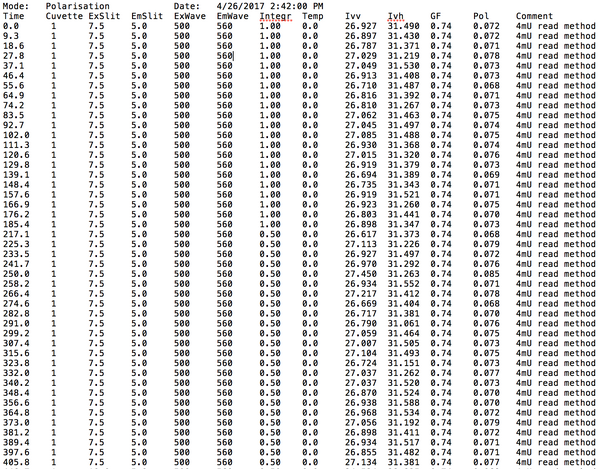
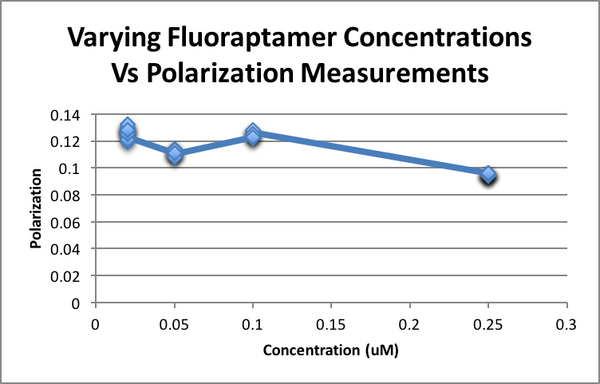
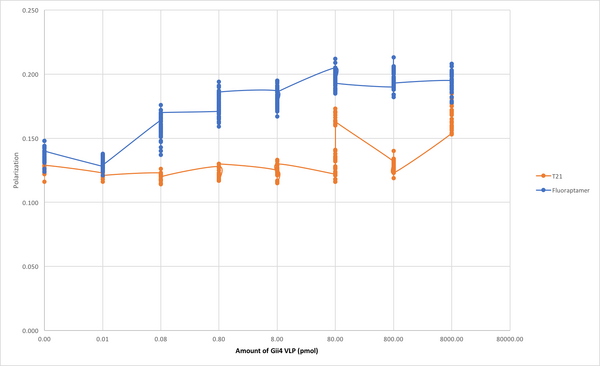
Biomod/2017/StJohns:Results
Results
NOTE: Unless otherwise stated, the agarose gels run (Fig. 12-19) were 0.5X TBE ~10mM Mg2+ 1% denaturing, with 0.5X TBE 10mM Mg2+ buffer, run at 25°C and 450-700 volthours.
All gel images have been uploaded in JPEG form, but TIFF forms can be made available upon request.
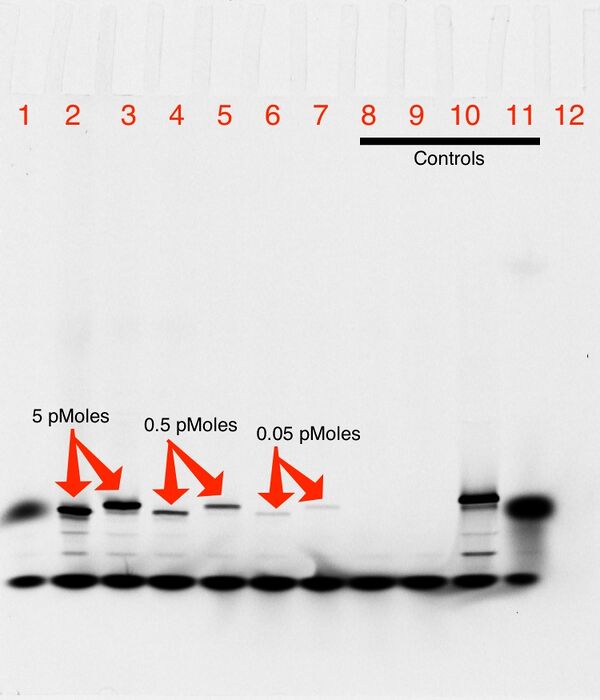
Effect of Trehalose on VLP Binding
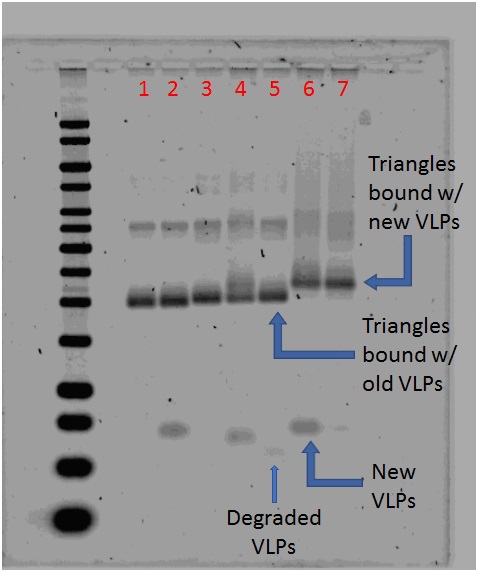
1% Agarose gel; 650 Vhrs
Lanes
- 0.05 pmol Triangle blunt
- 0.05 pmol Triangle blunt + 0.20 pmol T21 VLP
- 0.05 pmol Triangle sticky
- 0.05 pmol Triangle sticky + 0.20 pmol wild type VLP
- 0.05 pmol Triangle sticky + 0.20 pmol old T21 VLP
- 0.05 pmol Triangle sticky + 0.20 pmol new T21 VLP (fresh)
- 0.05 pmol Triangle sticky + 0.20 pmol new T21 VLP (1 week frozen)
Tightening of Bands as a Result of Removing Hinge Staples
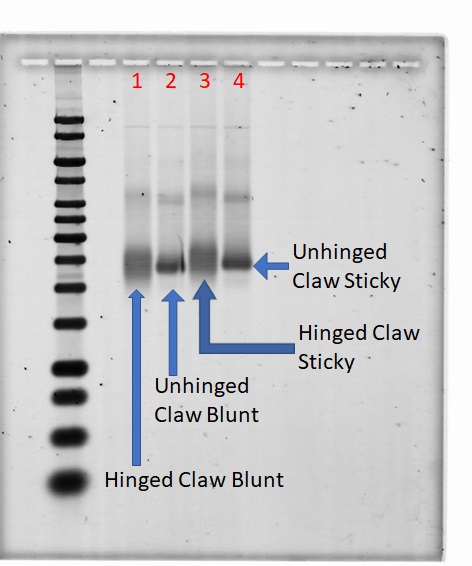
1% Agarose; 700 Vhrs
Lanes:
- 0.05 pmol hinged claw blunt
- 0.05 pmol unhinged claw blunt
- 0.05 pmol hinged claw sticky
- 0.05 pmol unhinged claw sticky
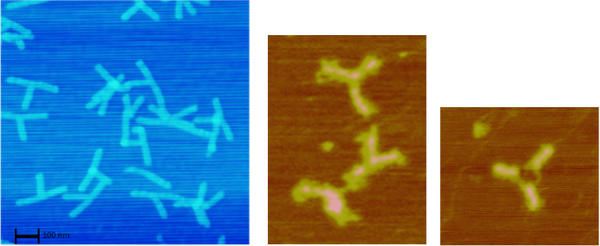
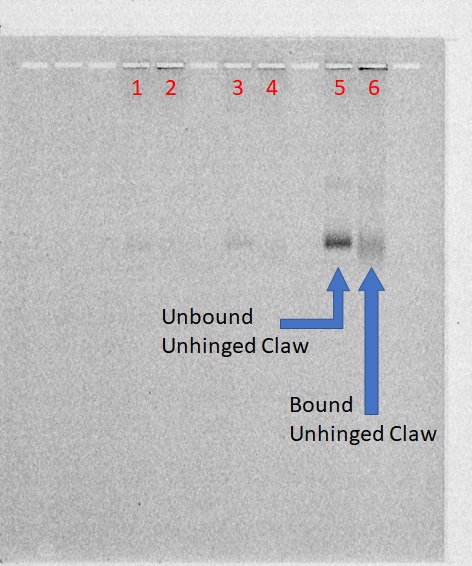
Distinction Between the Bound and Unbound Version of Unhinged Claw to VLP
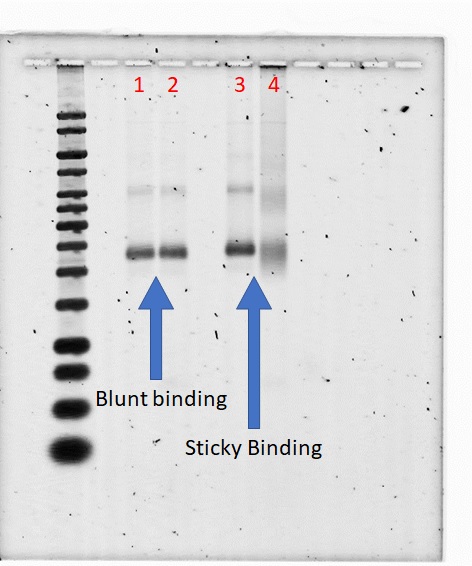
1% Agarose; 690 Vhrs
Lanes:
- 0.05 pmol unhinged claw blunt
- 0.05 pmol unhinged claw blunt + 0.20 pmol T21 VLP
- 0.05 pmol unhinged claw sticky
- 0.05 pmol unhinged claw sticky + 0.20 pmol T21 VLP
FRET Signaling of Bound and Unbound Claw
1% Agarose; 680 Vhrs
Lanes:
- 0.05 pmol unhinged claw sticky (donor only)
- 0.05 pmol unhinged claw sticky (donor only) + 0.20 pmol T21 VLP
- 0.05 pmol unhinged claw sticky (acceptor only)
- 0.05 pmol unhinged claw sticky (acceptor only) + 0.20 pmol T21 VLP
- 0.05 pmol unhinged claw sticky (donor+acceptor)
- 0.05 pmol unhinged claw sticky (donor+acceptor) + 0.20 pmol T21 VLP
Yield Produced by Cross-Linking
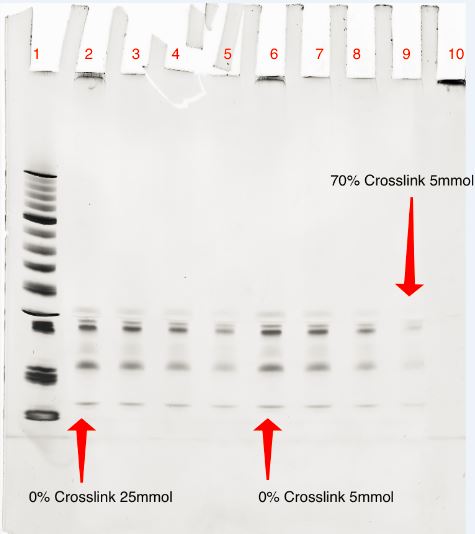
20% Denaturing Acrylamide; 400 Vhrs
Lanes:
- Marker
- 0.05 pmol Triangle + 25 mmol 8 MOP (Crosslinked 0min)
- 0.05 pmol Triangle + 25 mmol 8 MOP (Crosslinked 30 min)
- 0.05 pmol Triangle + 25 mmol 8 MOP (Crosslinked 90 min)
- 0.05 pmol Triangle + 25 mmol 8 MOP (Crosslinked 270 min)
- 0.05 pmol Triangle + 5 mmol 8 MOP (Crosslinked 0 min)
- 0.05 pmol Triangle + 5 mmol 8 MOP (Crosslinked 30 min)
- 0.05 pmol Triangle + 5 mmol 8 MOP (Crosslinked 90 min)
- 0.05 pmol Triangle + 5 mmol 8 MOP (Crosslinked 270 min)
- Plasmid
Increased Reaction Time Led to Increased Crosslinking Yield
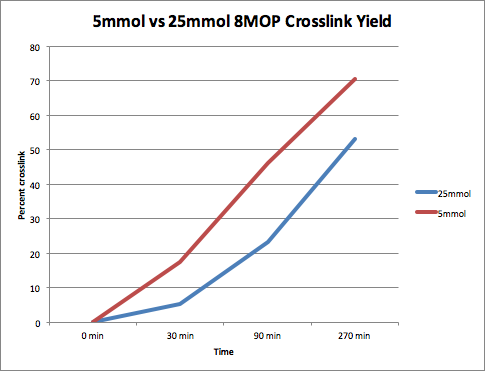
Binding triangle with fluorescently labeled DNA strand 40%
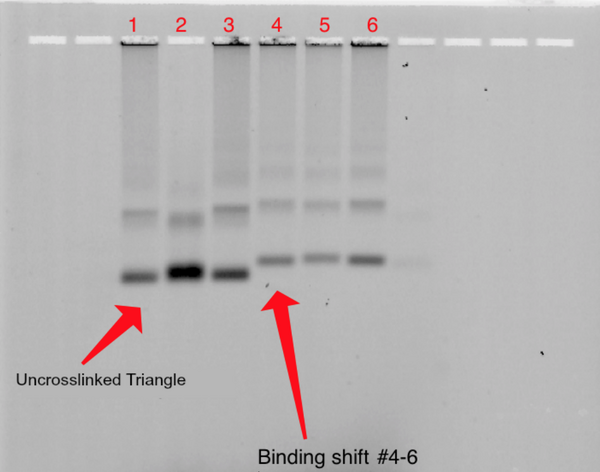
1% Agarose; 680 Vhrs
Lanes:
- ~0.05pm Uncrosslinked Triangle + T21 Fluorescent Strand
- ~0.05pm Uncrosslinked Triangle + T21 Fluorescent Strand
- ~0.05pm Uncrosslinked Triangle + T21 Fluorescent Strand
- ~0.05pm Crosslinked Triangle (270 min) + T21 Fluorescent Strand
- ~0.05pm Crosslinked Triangle (270 min) + T21 Fluorescent Strand
- ~0.05pm Crosslinked Triangle (480 min)+ T21 Fluorescent Strand
Purity Check and Quantifying Methylene Blue Strands
20% Acrylimide Gel; 794 Vhrs (Imaged using scan for MB before staining)
Lanes:
- 10 bp Ladder
- 5.0 pmol (TCA)7 MB
- 5.0 pmol T21 MB
- 0.5 pmol (TCA)7 MB
- 0.5 pmol T21 MB
- 0.05 pmol (TCA)7 MB
- 0.05 pmol T21 MB
- 5.0 pmol A21 C10
- 5.0 pmol (TCA)7 MS2
- 0.5 pmol Claw XII ATTO
- Denaturing Dye
- Blank
Quantifying Methylene Blue strands on Triangle
Prestain
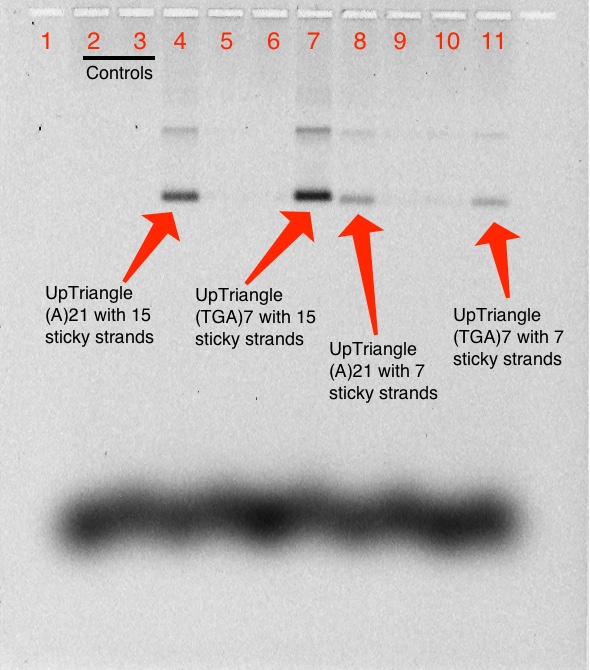
1% Agarose Gel; 487 Vhrs (Imaged using scan for MB before staining)
Lanes:
- 1kb Ladder
- 0.05 pmol Triangle Blunt + 5.0 pmol T21 MB
- 0.05 pmol Triangle Blunt + 5.0 pmol (TCA)7 MB
- 0.05 pmol Up Triangle(15) A21 + 5.0 pmol T21 MB
- 0.05 pmol Up Triangle(15) A21 + 5.0 pmol (TCA)7 MB
- 0.05 pmol Up Triangle(15) (TGA)7 + 5.0 pmol T21 MB
- 0.05 pmol Up Triangle(15) (TGA)7 + 5.0 pmol (TCA)7 MB
- 0.05 pmol Up Triangle(7) A21 + 5.0 pmol T21 MB
- 0.05 pmol Up Triangle(7) A21 + 5.0 pmol (TCA)7 MB
- 0.05 pmol Up Triangle(7) (TGA)7 + 5.0 pmol T21 MB
- 0.05 pmol Up Triangle(15) (TGA)7 + 5.0 pmol (TCA)7 MB
SYBR Stain
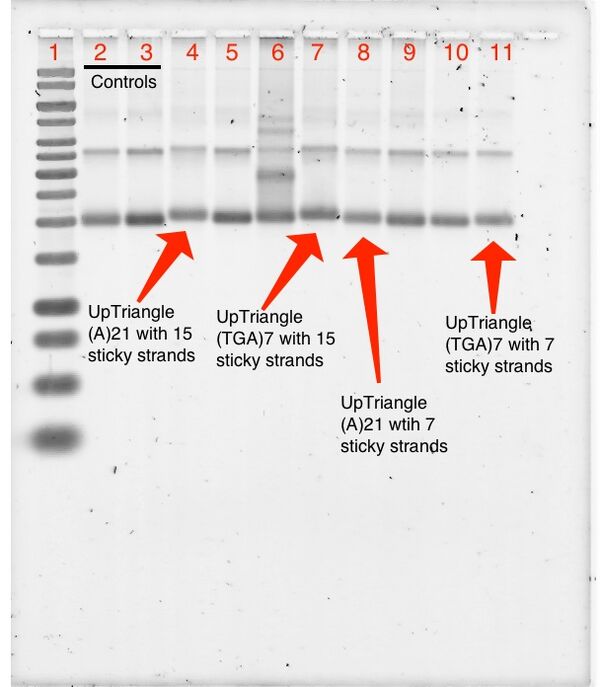
1% Agarose Gel; 487 Vhrs (SYBR stain)
Lanes:
- 1kb Ladder
- 0.05 pmol Triangle Blunt + 5.0 pmol T21 MB
- 0.05 pmol Triangle Blunt + 5.0 pmol (TCA)7 MB
- 0.05 pmol Up Triangle(15) A21 + 5.0 pmol T21 MB
- 0.05 pmol Up Triangle(15) A21 + 5.0 pmol (TCA)7 MB
- 0.05 pmol Up Triangle(15) (TGA)7 + 5.0 pmol T21 MB
- 0.05 pmol Up Triangle(15) (TGA)7 + 5.0 pmol (TCA)7 MB
- 0.05 pmol Up Triangle(7) A21 + 5.0 pmol T21 MB
- 0.05 pmol Up Triangle(7) A21 + 5.0 pmol (TCA)7 MB
- 0.05 pmol Up Triangle(7) (TGA)7 + 5.0 pmol T21 MB
- 0.05 pmol Up Triangle(15) (TGA)7 + 5.0 pmol (TCA)7 MB
Attachment of MB strands to Side-Triangle
Prestain
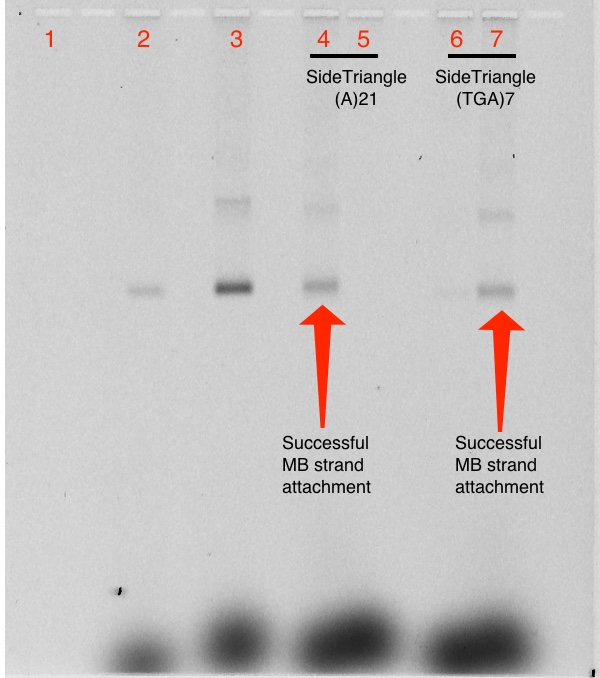
1% Agarose Gel; 581 Vhrs (Imaged using scan for MB before staining)
Lanes:
- 1kb Ladder
- 0.05 pmol Up Triangle(15) A21 + 5.0 pmol T21 MB
- 0.05 pmol Up Triangle(15) (TGA)7 + 5.0 pmol (TCA)7 MB
- 0.05 pmol Side Triangle(15) A21 + 5.0 pmol T21 MB
- 0.05 pmol Side Triangle(15) A21 + 5.0 pmol (TCA)7 MB
- 0.05 pmol Side Triangle(15) (TGA)7 + 5.0 pmol T21 MB
- 0.05 pmol Side Triangle(15) (TGA)7 + 5.0 pmol (TCA)7 MB
SYBR Stain
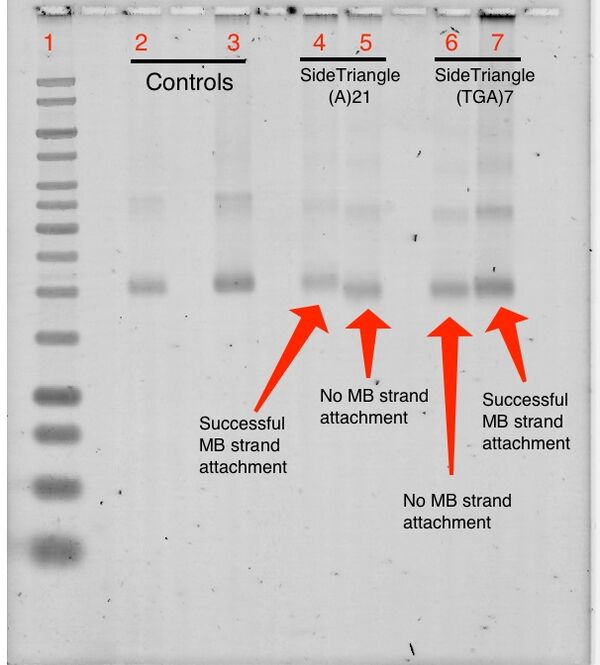
1% Agarose Gel; 581 Vhrs (SYBR stain)
Lanes:
- 1kb Ladder
- 0.05 pmol Up Triangle(15) A21 + 5.0 pmol T21 MB
- 0.05 pmol Up Triangle(15) (TGA)7 + 5.0 pmol (TCA)7 MB
- 0.05 pmol Side Triangle(15) A21 + 5.0 pmol T21 MB
- 0.05 pmol Side Triangle(15) A21 + 5.0 pmol (TCA)7 MB
- 0.05 pmol Side Triangle(15) (TGA)7 + 5.0 pmol T21 MB
- 0.05 pmol Side Triangle(15) (TGA)7 + 5.0 pmol (TCA)7 MB
Fluorescence Polarization Results