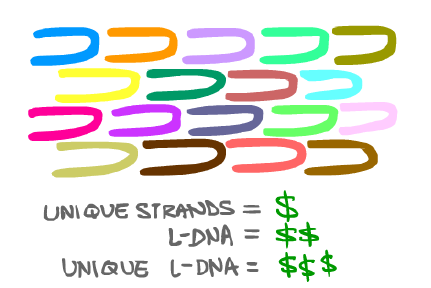Biomod/2012/Harvard/BioDesign/introduction
<html>
<head>
<link href='http://fonts.googleapis.com/css?family=Open+Sans' rel='stylesheet' type='text/css'>
</head>
<style>
/*BUT ACTUALLY*/ body {
font-family: 'Open Sans', sans-serif; overflow-y: scroll;
}
.container {
background-color: #ffffff; margin-top:0px
} .OWWNBcpCurrentDateFilled { display: none; }
h5 {
font-family: 'Open Sans', sans-serif; font-size: 11px; font-style: normal; text-align: center; margin:0px; padding:0px;
}
- column-content
{
width: 0px; float: left; margin: 0 0 0 0; padding: 0;
} .firstHeading {
display:none; width:0px;
}
- column-one
{
display:none; width:0px; background-color: #ffffff;
}
- globalWrapper
{
width: 900px; background-color: #ffffff; margin-left: auto; margin-right: auto
}
- content
{
margin: 0 0 0 0; align: center; padding: 12px 12px 12px 12px; width: 876px; background-color: #ffffff; border: 0;
}
- bodyContent
{
width: 850px; align: center; background-color: #fffffff;
}
- column-content
{
width: 900px; background-color: #ffffff;
}
- footer
{
position: center; width: 900px;
} @media screen {
body { background: #000000 0 0 no-repeat; /* changed default background */ }
}
- menu
{
position: fixed; float: left; width: 180px; padding: 10px; background-color: #FFFFFF;
}
- pagecontent
{
float: right; width: 620px; margin-left: 300px; min-height: 400px
}
- toc { display: none; }
/*Expanding list*/ ul { list-style: none; }
- exp li ul { display: none; }
- exp li:hover ul { display: block; }
- exp li a:active ul { display: block; }
a:link {color:#FF6060;} a:visited {color:#FF6060;} /* visited link */ a:hover {color:#B24343;} /* mouse over link */ a:active {color:#B24343; } /* selected link */
</style> </html>
Introduction
Background
In February 2012, Shawn M. Douglas, Ido Bachelet, and George M. Church at Harvard University demonstrated the use of DNA origami to create a drug-delivering nanorobot. This device carries with it remarkable advantages including cell-specificity, biocompatibility, and the ability to protect fragile, toxic, or previously undeliverable drugs.
Problem
While an exciting advancement in drug delivery, the nanorobot has a fundamental flaw: it is degraded by nucleases, enzymes found in the body which cut and digest DNA.
Professor Paul Rothemund, a well-known pioneer in DNA nanotechnology, noted in an accompanying Nature article that "[i]f these sorts of problems can be solved, then the nanorobots have a chance at becoming real therapeutics."
Inspired by the DNA nanorobot's potential as a future therapeutic, our team decided to tackle the issue of nuclease resistance.
Solution
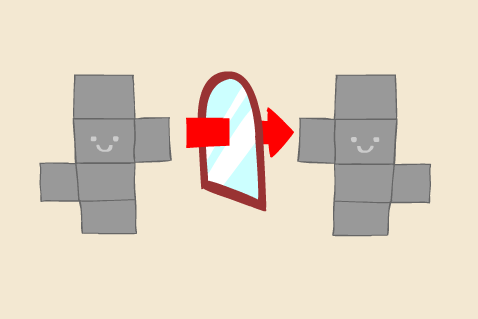
We focused on one possible method to create nuclease resistant structures: by using a different material. We decided to work with L-DNA as our enzyme-resistant nanomaterial. L-DNA, the mirror image of normal DNA, cannot be recognized and degraded by the chiral nucleases.
While exciting, the use of L-DNA alone is not the only solution. The DNA nanorobot, made with the DNA origami technique, requires the use of a long phage-derived scaffold strand (7,308 base pairs). Because L-DNA is completely synthetic, we cannot use a phage to make it. Furthermore, to synthesize L-DNA of this length would be extremely difficult as the error rate would be unacceptably high.
Confronted with this issue, our team decided to utilize Single-Stranded Tiles (SSTs), a different method of creating nano structures. In essence, the SST approach uses shorter, modular strands. The idea was created by Peng Yin in 2008 and explored by Bryan Wei et. al. in 2012.
Before we continue, here's a quick video on how the SST technique works, courtesy of the Wyss Institute at Harvard University.
<html>
<iframe src="http://player.vimeo.com/video/42849360?title=0&byline=0&portrait=0&badge=0" width="500" height="281" frameborder="0" webkitAllowFullScreen mozallowfullscreen allowFullScreen></iframe>
<a href="http://vimeo.com/42849360">How Single-Stranded Tiles (SSTs) Work</a>
</html>
Unlike DNA origami, the SST method only requires short, 42-mer DNA strands, allowing us to use L-DNA.
But this gives rise to another problem. To use L-DNA as the basis for any structure, we would have to use hundreds of unique L-DNA SSTs to provide the specificity that the SST technique requires. Unfortunately, the cost of synthesis rises with the number of unique strands. With L-DNA already expensive to synthesize, the cost of making any L-DNA SST structure is far too high to pursue development with the standard SST approach.
To solve this price issue, we needed to limit the number of unique strands. Our team focused on reducing the number of unique strands: we got it down to just two.
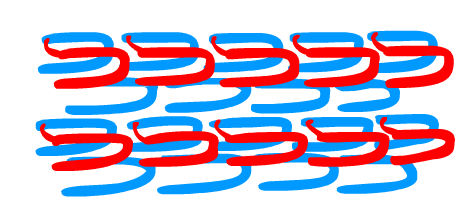
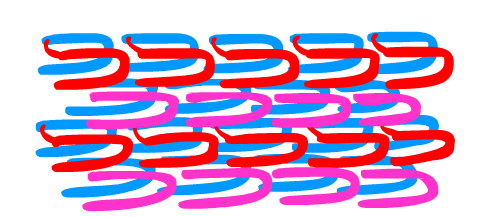
The solution involved more than just using two sets of strands which alone cannot make a defined and controllable structure like a nanorobot (Yin et al. 2008). We adapted a method of "templating", using unique D-DNA SST strands to form a template structure first, and then attaching L-DNA on top. In this manner, we were able to define and control the L-DNA SST structure.
To do this, we first attached L-DNA SST of one type to the template. We then added our complimentary second L-DNA SST. This allowed for us to create a defined and controllable L-DNA SST structure.
The result of our project is a method that balances self-assembly with nuclease resistance. With this technique, we can create a size-determined L-DNA sheet - the first step towards a nuclease resistant nanorobot for drug delivery.
Project Goals
Over the summer of 2012, our team successfully achieved all of our project goals:
- Create a modified D-DNA template
- Bind one set of L-DNA strands to the template
- Purify the scaffolds away from the excess unbound L-DNA SST
- Bind second type of L-DNA SST onto purified scaffolds
- Document the cheap, nuclease-resistant L-DNA nanostructures made using SSTs and an elegant templating method.