BME100 s2017:Group9 W8AM L2
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | |||||||
OUR TEAM
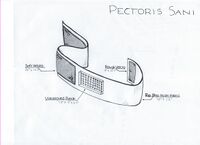
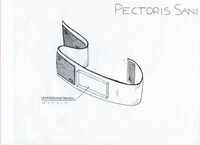
LAB 2 WRITE-UPDevice Image and Description
Technical and Clinical FeasibilityTechnical Feasibility The technologies that are needed for this device to function properly are an ultrasound patch and bluetooth technology. The patch is incorporated into a band made with a strong material made of nylon, neoprene and lycra. The device will also use velcro to attach and stay snuggly attached around the body. The challenges that come out of this device are mostly to do with the ultrasound patch.The first and most important function of the device is the readings that the ultrasound patches provides. Women will be reliant on these devices to warn them of changes in their breast density so that they can seek medical attention in time to increase their survival rate. The reliance on the device means the readings must be valid and precise. Ultrasound devices provide false negatives at a rate of 1/2500 readings. (1) This rate is fairly low but with a less established device as the ultrasound patch it is possible that this rate will be higher. The purpose of our device is not to monitor breast density once every year but more often. Hopefully, having women take readings once a month the diagnosis will be based on changed in the women's unique body type and thereby will be more reliable. Handheld ultrasound readings can be more effective but continual readings over time can trigger women to seek further scanning and medical testing before their scheduled annual check ups if a severe change is detected. In order to determine if the readings are showing change in density the ultrasound readings must be monitored by a professional radiologist to determine what the results mean in terms of possible cancerous tissue. A registered technician is able to determine what changes are representative of cancer and which ones are natural changes in the body. (2) This area of the device must be able to send the results to a handheld device and then can be relayed to a doctor's office. The challenge here is ensuring the information is relayed correctly. The handheld device will have to be built with wifi technology to send the results to a radiologist. There are several things that can go wrong in this device.
(1) "Breast Screening." False Negative Results | Topics, Breast Screening, Cancer, People's Experiences. DIPEx, n.d. Web. 08 Feb. 2017. <http://www.healthtalk.org/peoples-experiences/cancer/breast-screening/false-negative-results> (2) Halls, Steven. "Ultrasound Scan for Breast Cancer Screening - Moose and Doc." Breast Cancer - Moose and Doc. N.p., 06 Feb. 2017. Web. 08 Feb. 2017. <http://breast-cancer.ca/ult-bens/>. Clinical Feasibility The process for the device is noninvasive and inexpensive. Especially for people who are predisposed to breast cancer, the device will be very helpful for early diagnosis. The creators are foreseeing a high chance in the increase in clinical feasibility. Unfortunately, false positives are to be looked out for. The way that this device will be tested in a clinical setting will be very simple. Very much like the testing t=for the Automated Breast Ultrasound (1) the patients will be chosen from a range of ages (18-90) and will be monitored for six months to a year or more, if necessary. Similar technologies have taken much longer to complete clinical trials when new technologies are being introduced. For example, one study has taken closer to 3 years to complete trials and have not yet completed their research on a new ultrasound device to detect kidney or liver lesions. (3) The people will be tested once a month by both the current ultrasound technologies and then again by our device. The readings will be taken by different doctors so that they will be blind to what the other test has shown. The device should work in a clinical setting as it is just a new application for existing technology. The issue being tested at the clinical level will test more of if this new application is reasonable and efficient. A similar technology of non hand held ultrasound breast cancer screening was tested in 2016 and proved that their technology was effective at determining breast caner.(2) The trials took two years to be completed and approved. They tested 501 women between the ages of 18 and 90 with both a handheld ultrasound and the ABVS technology to see if both devices produced the same results. There were no risks associated with this device as it does not permit radiation that could be harmful to patients. (1) (1) Mendelson, Ellen. "Lesion Detection of Automated Breast Ultrasound Compared With Handheld Physician-performed Breast Ultrasound." Lesion Detection of Automated Breast Ultrasound Compared With Handheld Physician-performed Breast Ultrasound - Study Results - ClinicalTrials.gov. Northwestern University, n.d. Web. 08 Feb. 2017. <https://clinicaltrials.gov/ct2/show/results/NCT02310776?term=ultrasound%2Bpatches&rank=7>. (2) Boyd. "ACUSON S2000 ABVS Ultrasound System, HELX Evolution with Touch Control." Siemens Healthneers. N.p., 2016. Web. <https://www.healthcare.siemens.com/ultrasound/breast-care/acuson-s2000-abvs-ultrasound-machine>. (3) Stanford University. "Ultrasound Elastography in Diagnosing Patients With Kidney or Liver Solid Focal Lesions." Ultrasound Elastography in Diagnosing Patients With Kidney or Liver Solid Focal Lesions - Full Text View - ClinicalTrials.gov. National Cancer Institute, 26 Jan. 2017. Web. 08 Feb. 2017. <https://clinicaltrials.gov/ct2/show/study?term=ultrasound%2Bpatches&rank=11>.
Market AnalysisValue Creation Manufacturing Cost Works Cited "[1]. Rip Stop Nylon Fabric." Joann.com. N.p., n.d. Web. 08 Feb. 2017. "[2]. NanoVibronix." PainShield® Kit | Products | NanoVibronix. N.p., n.d. Web. 08 Feb. 2017. "[3]. Insignia™ - Bluetooth 4.0 USB Adapter - Black." Best Buy. N.p., 07 Dec. 2016. Web. 08 Feb. 2017. "[4]. 24 X 1 Inch Cinch Straps - 5 Pack." Secure™ Cable Ties. N.p., n.d. Web. 08 Feb. 2017. "[5]. The Italian Model of Management." Google Books. N.p., n.d. Web. 08 Feb. 2017. Sales Price Works Cited "[1]. Mammogram Guidelines: What's Changed?" Mayo Clinic. N.p., n.d. Web. 08 Feb. 2017. "[2]. Breast Cancer Under 40: Early Detection." Cleveland Clinic. N.p., n.d. Web. 08 Feb. 2017. "[3]. How Much Does a Mammogram Cost? - CostHelper.com." CostHelper. N.p., n.d. Web. 08 Feb. 2017. [4]. Legislatures, National Conference of State. "Health Insurance: Premiums and Increases." Health Insurance: Premiums and Increases. N.p., n.d. Web. 08 Feb. 2017. "[5]. How Much Does Health Insurance Cost Without A Subsidy?" EHealth Insurance Resource Center. N.p., 30 Jan. 2017. Web. 08 Feb. 2017. Market Size Works cited [1]. "How Common Is Breast Cancer?" American Cancer Society. N.p., n.d. Web. 10 Feb. 2017. [2]. "U.S. Population." US Population, Women's Health USA 2012. N.p., n.d. Web. 10 Feb. 2017. [3]. "American Cancer Society Guidelines for the Early Detection of Cancer." American Cancer Society. N.p., n.d. Web. 10 Feb. 2017.
Fundability DiscussionTechnical feasibility: 2- Some challenges but will be overcome with time and within a reasonable cost. This rating was determined for this device because the technology is taking a new device and altering it. While ultrasound patches do exist they are most commonly used to treat pain and will likely need to be altered to act as effectively as a handheld ultrasound device. Should the research show that the current technology cannot be transformed to this function a new piece of technology will need to be developed and that will cost a considerable amount of money and would most likely extend the process by quite a long amount of time. Clinical feasibility: 2- Clinical success is likely but will require special expertise and/or research This device claims to be able to treat all women and men and in order to prove that this is possible many different ages and body sizes will need to be tested. It does not necessarily need to be tested for an exorbitant amount of time but the device should be able to last for several years at promised function which will require some form of test to ensure the quality of readings does not go down over time. Overall fundability: Score: 16 Customer Validation: 1 No customers feedback to determine interest or validation. Market size: 2 If current market is saturated the market size will be large. Competition: 1 There are many different companies working on ways to find breast cancer early. IP Position:1 No patents have been issued at this point. Technical Feasibility: 2 Some research will be necessary but it is not exorbitant. Regulatory Pathway: 2 Clinical data will be needed but not a completely new device. Clinical Feasibility: 2 There will need to be tests to determine function and ability to diagnose. Reimbursement: 1 New application to device.
|
|||||||