BME100 s2017:Group2 W8AM L2
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | ||||||||||||||||||||||||||||||||||||||||||||||
OUR TEAM
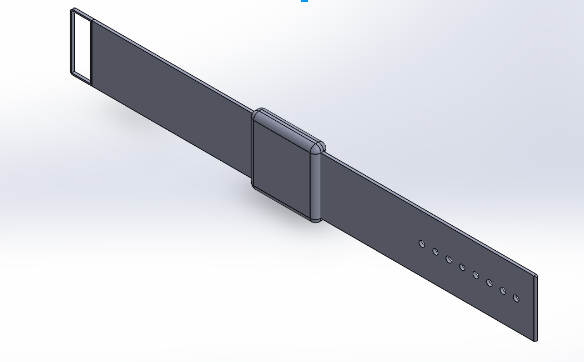
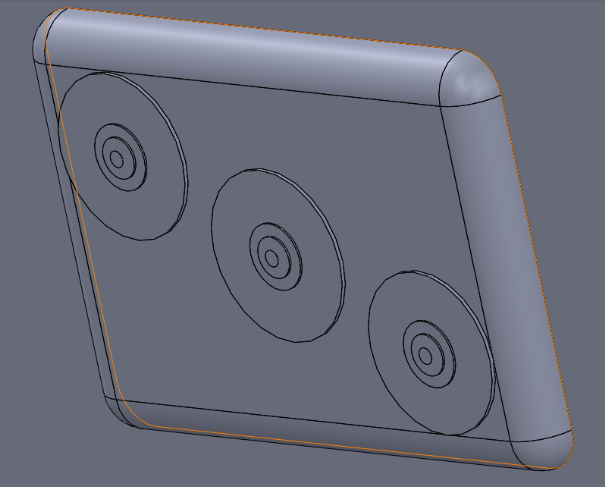
LAB 2 WRITE-UPDevice Image and Description
Technical and Clinical FeasibilityTechnical Feasibility The technologies required are basic capabilities of a smartwatch, dry electrode sensors to accurately measure heart rate, and a system to contact paramedics from the watch. The challenges include that the dry electrodes require a stiff material that could move on the skin, making it hard to get an accurate reading. Another problem is that dry electrodes tend to be very expensive and have high power consumption. The system also produces quite a bit of noise. The dry electrodes could irritate the skin of the user, the electrodes could be bumped or moved, causing interference to the heart rate reading, or the system to contact paramedics could malfunction and the patient would not receive the necessary medical attention.
Many dry electrode systems have been tested but none have been universally accepted. The use of flexible polymers, a single chip instrumentation amplifier that uses less power and noise, and placing the electrode on the wrist in a rubber band could help solve the problems others encountered when developing a successful dry electrode. If all these changes are used, it should work in clinical trial. A major risk is that the resistance from the dry electrode could cause irritation to the skin. Many people have tested dry electrode systems since 1973. In 2008, polydimethylsiloxane was used and worn around the wrist and did not show signs of irritation over a period of one week and also, showed sustainability without the chemical of the skin interfering with it. A version of the electrode using microneedles was tested but when the needles broke it caused infection and inaccurate readings. One of the major advantages of having a dry electrode system is that it is exceptionally more accurate than a photoplethysmography (PPG) sensor which is the technology used in the designs of leading competition.
Market AnalysisValue Creation The prototype creates value for the customer because it combines the abilities of two devices into a single device. Since this device is marketed toward older individuals or individuals at risk for health emergencies, they may already be looking at acquiring two devices. Those who are interested in having these technologies in a single stylish device would save money as well as body landscape. A device with these combined services would also encourage elderly individuals to stay at home instead of moving into retirement homes. The dry electrode system could also monitor heart rate more accurately than other, similar devices. And by skipping the middleman-like response center and going straight to the local emergency center like a phone call does, total cost for the customer will be reduced for this technology, as well as eliminating the idea of a contract and monthly bill.
Dry Electrode Cost: $2.50 per electrode x 3 = $7.50 Watch Design Cost: about $76.50 (direct materials and manufacturing) MCU,32-Bit ARM Cortex-M3, 48MHz, 128KB RAM, 8-Channel 12-Bit ADC,93 GPIOs-SILICON LABORATORIES INC ($9.53) Display Window 1.26" Diagonal ($20.50) Display Module 1.26" Diagonal ($7.05) Bluetooth USB Dongle Bluetooth V4.0, Contains Texas Instruments CC2540 - (Qty: 1) ($1.84) Accelerometer / Gyroscope / Magnetometer 9-axis Motion Tracking, INVENSENSE INC-(Qty: 1) ($6.62) Amplifier, Op Amp 1.8V, 7MHz, Low Offset, Rail-to-Rail, TEXAS INSTRUMENTS (Qty: 2) ($0.36 x 2 = $0.72) GPS Receiver Single Chip, 66-Channel GPS GNSS / QZSS / SBAS, ARM 7 CPU, w/ Built in LNA, w/ 16 GPIOs - MEDIATEK INC-(Qty: 1) ($39.95) Total Cost= $84.00 Sales Price We believe our ASP should be $200.00 because it fits the current selling market for similar devices and gives us a good return for the money we put into production.
100% Market Size: (27,612,000 people) x ($116) = $3,202,992,000 5% Market Size: (1,380,600 people) x ($116) = $160,149,600 (CDC, 2017)
Fundability Discussion
|
||||||||||||||||||||||||||||||||||||||||||||||