BME100 f2017:Group9 W1030 L6
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | ||||||
|
OUR TEAM
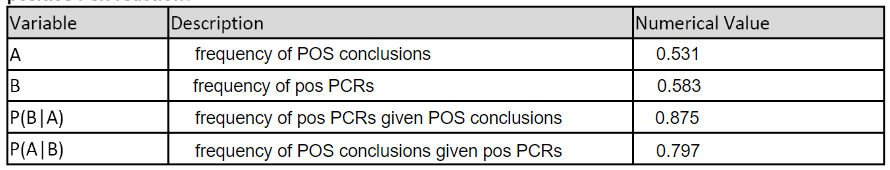
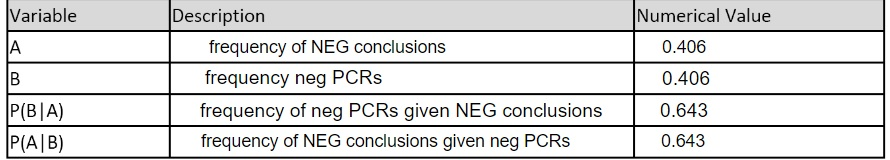
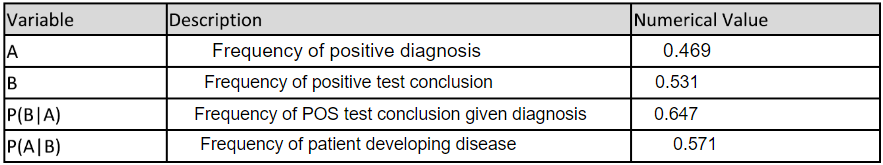
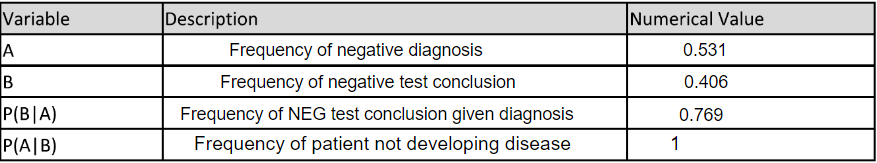
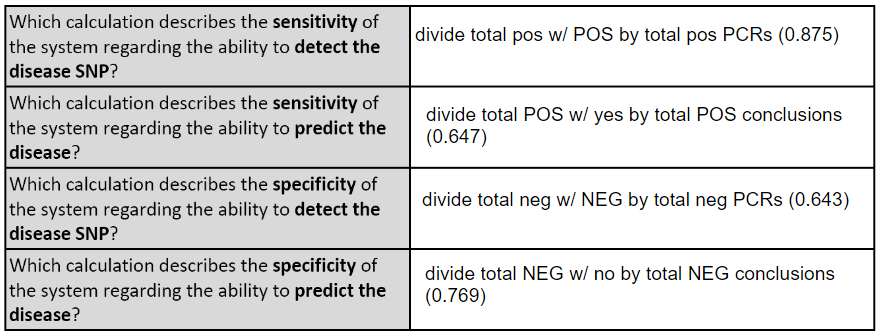
LAB 6 WRITE-UPBayesian StatisticsOverview of the Original Diagnosis System BME 100 students began by receiving two different DNA samples from two separate patients. PCR master mix and the forward and reverse primers were then added to the samples as well as the positive and negative controls. There were a total of 8 microtubes that were then placed into the PCR machine. One tube had the positive control, one tube had the negative control, three tubes contained patient one's DNA, and the final three tubes contained patient two's DNA. After the PCR machine ran to completion the tubes were removed and buffer was added to each sample. 160µL drops of each sample were then placed in the middle of a glass slide. This slide was then placed on top of a fluorimeter in a dark box where a picture was taken in order to show the light intensity of solely the singular drop. If the drop glowed green to the naked eye then the sample was classified as containing the disease-associated SNP. ImageJ was also utilized to determine the exact presence of the green color and make the data quantitative. Multiple pictures and trials(three pictures per drop and three drops per patient) were used in order to limit human error in the experiment. Challenges that were encountered included the placement of the drop in the exact center of the glass slide; therefore, our new design of the fluorimeter accounted for this possible error and made the placement more standardized. What Bayes Statistics Imply about This Diagnostic Approach
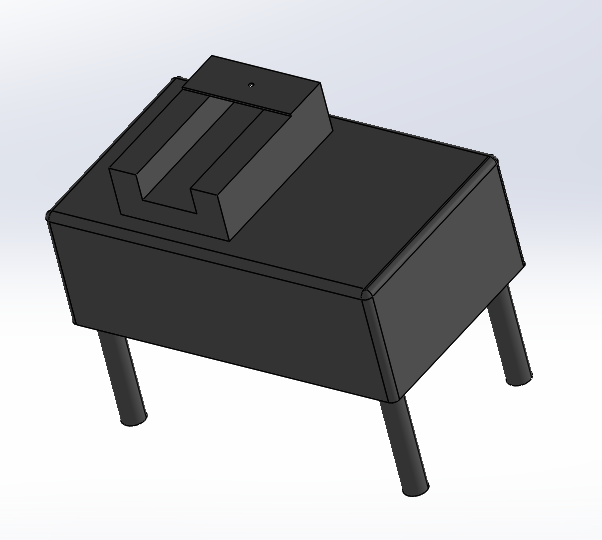
Intro to Computer-Aided Design3D Modeling The software used to design our device was SolidWorks. This software allowed us to add measurements and details to a three-dimensional model of our device. It includes features that change the material of the device or the background, which gives a more accurate display of the different parts. It effectively communicates the main idea of our device to others, specifically investors and consumers of the product. In order to adequately utilize this software, some basic skills such as sketching, editing dimensions, extruding, and cut extruding are needed. With these skills, SolidWorks provides a simple and quick way to accurately display a proportional model of any desired part or design. Our Design
Feature 1: ConsumablesIn the new Open PCR kit, specific consumables will be included in the package. Such consumables will include small plastic microtubes, a pipettor, and various reagents of PCR mix and primers. The microtubes will be adjusted from their current state by being pre-labeled with the titles "negative control", "positive control", and the various possibilities of patient numbers to ensure that samples remain distinct from each other and are easy to identify. The pipettor, however, will be adjusted for easier use by adding user friendly directions directly on the device such as diagrams of how to properly utilize the first and second stop mechanisms when retrieving and transporting PCR solutions. The reagents will remain the same as those in current kits since they are necessary for DNA replication. These alterations in the consumables will decrease human errors made in the preparation of PCR and will be more user friendly. Feature 2: Hardware - PCR Machine & FluorimeterThe current PCR machine will not be changed from current models. This is because its existing state is generally compact, accurate, and efficient once the samples have been prepared and placed inside the Open PCR machine. The PCR machine does require an external power source (wall outlet) that could be considered inconvenient. However, an alternative power source would be dependent on the user's preference and can be altered without affecting the integrity of the PCR machine. Therefore, it is not necessary to change the power source (i.e. plug to batteries) as this can lead to unnecessary costs of continuously purchasing batteries without altering the outcome of the PCR process. The current fluorimeter was susceptible to human error, mainly through placement of aqueous samples and alignment with the phone. The new fluorimeter is meant to compensate for that. The new fluorimeter possesses detachable leg stands and a place holder for the micro pipette. The legs extend out and have a notch system for length and lock. This is to compensate for varying phone size. This is better than having to place the current fluorimeter on various objects to raise height. The place holder is located on the top of the fluorimeter and above the area where samples are placed. It is detachable and has a designated hole in the center to place the micro pipette through. Using this will allow the sample to be dropped exactly on the center. This is to prevent shakiness from the hand and placing the sample off center.
| ||||||