BME100 f2017:Group5 W0800 L1
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | |||||
OUR TEAM
LAB 1 WRITE-UPHealth Care IssueAir Pollution is killing over 500,000 people in China every year due to the extreme amount of pollutants found in the air. This is because China produces and consumes as much coal as almost the whole world combined. Coal inhalation can lead to things such as: bronchitis, emphysema, and asthma. In severe cases it can lead to things like black lung, lung cancer, and even death[3]. According to the EIA (The US Energy Information Administration) China’s coal consumption has risen by over 2.3 billion tons in the past 10 years.[4] The solution we have to this problem is an inhalant that will target specific toxins in the lungs and begin to break those toxins down, allowing macrophages to go in and begin the phagocytosis stage where they would finish breaking down these toxins. The device would most likely be administered at a hospital or outpatient clinic by a professional.
Customer ValidationThe patients of our product would be the employees of Shenhua Group, China Coal Group, Shaanxi Group and Chemical Industry, and Shanxi Coking Coal Group. These are the people who are affected by the air pollution the most and would have the most to gain from our product.[21] This is because they are the ones who are breathing in all the air pollution in day after day without any protection or treatment. The physicians that would administer our product would be the pulmonologists at hospitals that treat the people who have been affected by air pollution. These doctors gain more knowledge and a better product for treating people whose lungs have been damaged because of air pollution. The providers of our product would be the nurses and doctors at the following hospitals, Guangdong General Hospital, General Hospital of Tianjin Medical University, Beijing Jishuitan Hospital and the Chinese General Hospital and Medical Center. These hospitals are the main ones for treating people with lung diseases caused by air pollution. [22] The payers would be the insurance agents that deal with air pollution claims such as, China Life Insurance Co., Ping An of China, China Pacific Insurance, and the People’s Insurance Company of China Group. [21] The purchasers of our product would be companies such as, Medtronic, Quest Diagnostics, Blue Cross Blue Shield, Bio-reference Laboratories, and LabCorp. They would benefit from purchasing our product because it could help end some diseases of the lungs caused by air pollution along with treating previous cases of lung diseases.
CompetitorsComparison of existing medical solutions: Our solution is designed to clean the lungs, repair and reverse the damage caused to the lungs by coal pollutants without affect the immune system of the patient. Our solution is unique because instead of addressing the symptoms of the problem, our solution addresses the root of the problem to make the patient healthier. This makes our product a long term solution. We aim to make our solution as affordable, accessible, and as easy to use as possible for those who suffer asthma and pneumoconiosis in China due to their working conditions.
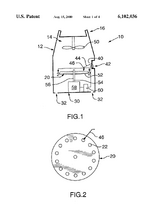
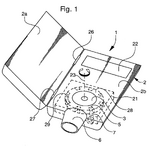
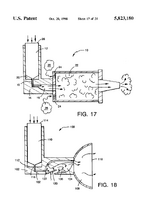
IP PositionMask patents: US4141703A, “Air-pollution filter and face mask”, 1997-03-04, “A flexible polymeric mask, which covers the mouth and at least the lower part of the nose, has exhale-valve means and vertical supporting means for air-intake filter means. The filter means provides a wearer of the mask with air which has passed, in sequence, through, e.g., porous foam, activated charcoal, filter paper, absorbent cellulose and gauze.”[5]
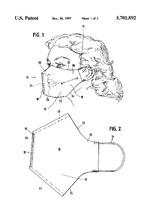
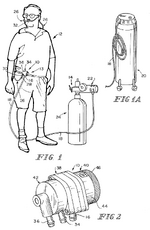
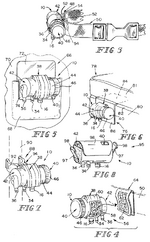
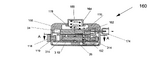
US5701892A, “Multipurpose face mask that maintains an airspace between the mask and the wearer's face”, 1995-12-01, “A multipurpose face mask made of supple material covers the nose, mouth, and chin with a two sided chamber held away from the entrance of the nostrils and the mouth by a rigid support attached inside the vertical front fold. This rigid support makes possible the use of a wide variety of soft materials in one or more layers, which may serve to filter dust, pollen, mold, dander, powder, and other common airborne particles, and/or to warm and humidify cold, dry air. For versatility in purpose, a disposable version may fit inside a reusable version. The cold weather version may have air holes in the outer layer. This device of supple material can be made in several sizes and rolled to fit in a pocket or purse and has an attractive, lean appearance with potential for embellishment. This invention in its many forms enhances the lives of people with respiratory disorders or professions which require respiratory protection.”[7] Oxygen tank patents: US6394088B1, “Oxygen delivery system with portable oxygen meter”, 1999-11-05, “An oxygen-delivery system comprises a portable oxygen meter including a low-pressure oxygen inlet, a low-pressure oxygen outlet, and an exhale-inhale sensing port, an oxygen-supply source including a discharge outlet and configured to discharge low-pressure oxygen through the discharge outlet, a flexible supply tube arranged to conduct low-pressure oxygen from the oxygen-supply source into the portable oxygen meter through the low-pressure oxygen inlet, and a nasal cannula coupled to the low-pressure oxygen outlet and the exhale-inhale sensing port. The portable oxygen meter further includes a pneumatic demand oxygen conserver including a diaphragm valve member, control means coupled to the exhale-inhale sensing port for causing the diaphragm valve member to move to a flow-delivery position in response to inhalation of a patient and to a flow-blocking position in response to a lack of inhalation by the patient during exhalation through the nasal cannula, and an oxygen flow passage arranged to pass low-pressure oxygen through an oxygen flow-metering aperture to meter low-pressure oxygen outlet at a selected oxygen flow rate. The oxygen-delivery system further comprises a mount including a flange coupled to the portable oxygen meter and a clip adapted to be coupled to an item of clothing worn by the patient.”[8] Inhaler patents: US6051566A, “Anti-reactive anti-asthmatic activity of non-steroidal anti-inflammatory drugs by inhalation”, 1991-03-02, “Non-steroidal anti-inflammatory drugs proved to be effective drugs for combating and preventing asthma if administered by inhalation. Useful are for instance acetylsalicylic acid, and indomethacin, and ketoprofen, and tenoxicam. Suitable pharmaceutical compositions for inhalation are aerosols with a particle size of from 0.5 μm to 7 μm which enter the compartments of the lungs. Aqueous or aqueous-organic solutions or suspensions can be nebulized in combination with propelling agents, or mixtures of powders with microfine drugs can be administered using inhalers. These pharmaceutical compositions may comprise appropriate additives, in order to improve their relevant pharmaceutical properties.”[9] US6102036A, “Breath activated inhaler”, 1998-07-30, “A method of assisting a person to withdraw from cigarette induced nicotine dependency, comprising introducing a predetermined dose of a non-pressurized, particulate medicament comprising at least one nicotine formulation suitable for absorption into the bloodstream of the person through the alveoli and small airways of the person's lungs into a breath activated inhaler for use by said person as a cigarette substitute. A breath activated inhaler which may be used in accordance with this method is also disclosed.”[10] EP0923957A1, “Liquid droplet spray device for an inhaler suitable for respiratory therapies”, 1998-06-23, “The liquid droplet spray device (5) comprises a micromachined substrate (8, 18, 18a; 8a, 18, 18a), a space (9) within the substrate for containing a liquid substance (4), means for supplying (7) the liquid (4) to the space (9), and outlet means (13, 14, 15) for ejecting the liquid as a mono-dispersive droplet spray when the liquid undergoes a vibration, The outlet means comprises a pyramid-shaped tapered cavity (13) which is micromachined in the space (9). The outlet means further comprises at least one output nozzle (14) and output channel (15) connecting the tapered cavity (13) to the outlet nozzle (14), the outlet nozzle (14) and the output channel (15) having a non-tapered vertical shape. Thanks to such a device, the exact amount ejected may be determined accurately which allows for a predicted lung deposition of the droplets by using appropriate control means adapted to the kind of patient.”[11] US8037880B2, “Dry powder inhaler”, 2006-04-07, “A new dry powder inhaler is developed as a pulmonary medicine delivery device for dispersing precise tiny dosages (10 μg-50 mg) of pure carrier-free ultra-fine powdered medicament (<5 μm aerodynamics particle size) into a patient's lung. The powder is drawn from the blister cell and dispersed through an outlet tube assisted by two air streams. The first air stream goes through a the blister cell from its upstream side, to significantly fluidize the medicament in the dose to flow upward. The second one extracts the fluidized powder from downstream of the blister cell for further deagglomeration and dispersion of the medicament powder by shear force. The rotating multi-dose blister can hold up to 60 doses, which are pre-metered with pure ultra-fine powdered medicament. So that it has higher drug loading capability in small volumes, compared to most current dry powder inhalers, which usually use some excipient. The inhaler efficiently disperse the aerosolized medicament in the air stream to the deep interior of patient's lung. The fine particle fraction (<4.7 μm) is reported to reach as high as 80% using this inhaler.”[12] Asthma medication patents: US20100008997A1, “Compositions and methods for treating asthma and other lung disorders”, 2009-05-01, “Provided are compositions and methods for treating lung or respiratory disorders or conditions characterized by airflow obstruction or limitation, or a symptom thereof (e.g., asthma, rhinitis, allergic rhinitis, and chronic obstructive pulmonary disease (COPD) and COPD-associated conditions (e.g., bronchitis, emphysema, asthma), emphysema, pneumonia, bronchitis, influenza, SARS, tuberculosis, and whooping cough (pertussis), and the like) in a subject in need thereof by administering a therapeutic composition comprising at least one electrokinetically altered fluid (gas-enriched (e.g., oxygen-enriched) electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures as disclosed herein. In certain aspects, the methods comprise regulating intracellular signal transduction by modulation of at least one of cellular membranes, membrane potential, membrane proteins (e.g., membrane receptors, (e.g., to G protein coupled receptors, and intercellular junctions)). Additional aspects include therapeutic compositions, and combination treatment methods comprising administration of electrokinetically generated fluid in combination with at least one additional therapeutic agent.”[13] US20080276935A1, “Treatment of asthma and chronic obstructive pulmonary disease with anti-proliferate and anti-inflammatory drugs”, 2008-06-09, “Embodiments of the present invention provide a method for treatment of respiratory disorders such as asthma, chronic obstructive pulmonary disease, and chronic sinusitis, including cystic fibrosis, interstitial fibrosis, chronic bronchitis, emphysema, bronchopulmonary dysplasia and neoplasia. The method involves administration, preferably oral, nasal or pulmonary administration, of anti-inflammatory and anti-proliferative drugs (rapamycin or paclitaxel and their analogues) and an additive.”, no design images[14] Mast cell stabilizer patents: US20060228306A1, “Combination antihistamine and steroid medication”, 2006-03-07, “The invention provides a topical pharmaceutical composition for application to the nasal or ocular mucosa which comprises (1) a pharmaceutical excipient suitable for topical administration, (2) an antihistamine drug and (3) a mast cell stabilizer, a non-steroidal anti-inflammatory drug, a phosphodiesterase inhibitor, an anti-IgE agent, heparin, a topical steroid or a leukotriene blocker. ”, no design images[15] WO2005063732A1, “Compounds and methods for the treatment of asthma”, 2004-12-13, “Compounds and methods for the treatment of asthma are disclosed. The methods involve mast cell stabilization together with selective inhibition of iNOS. The compounds are combinations of a mast cell inhibiting moiety and an inhibitor of iNOS.”, no images[16] Immunomodulator patents: US5823180A, “Methods for treating pulmonary vasoconstriction and asthma”, 1995-04-03, “This invention is a method for treating or preventing bronchoconstriction or reversible pulmonary vasoconstriction in a mammal, which method includes: a) causing the mammal to inhale a therapeutically effective amount of gaseous nitric oxide, and b) introducing into the mammal a therapeutically effective amount of phosphodiesterase inhibiting compounds; and an inhaler device (1) containing nitric oxide gas, and a phosphodiesterase inhibiting compound (106).”[17] Bronchodilator patents: US08317407, “Combination of a bronchodilator and a steroidal anti-inflammatory drug for the treatment of respiratory disorders”, 1994-10-03, “Effective amounts of formoterol and/or a physiologically acceptable salt and/or solvate thereof and budesonide are used in combination for simultaneous, sequential or separate administration by inhalation in the treatment of an inflammatory respiratory disorder, such as asthma.”, no images[18] US20020076382A1, “Formulations of mometasone and a bronchodilator for pulmonary administration”, 2001-08-01, “A pharmaceutical formulation is provided for pulmonary drug administration of a bronchodilator, a corticosteroid and an optional pharmaceutically acceptable carrier. In addition, methods for using the formulation to treat bronchodilator/corticosteroid-responsive conditions, diseases or disorders are provided, as are drug delivery devices and dosage forms for housing and/or dispensing the formulations.”[19] US5340587A, “Liposome/bronchodilator method & System”, 1989-06-13, “A method and system for treating bronchial constriction. The system includes a liposome composition containing a β2 -adrenoreceptor agonist in a liposome-entrapped form, and a device for aerosolizing a metered quantity of the composition. The drug in liposome aerosol form is substantially more effective in bronchodilation, over a substantially longer time, than the same amount of drug in free-drug aerosol form.”, images of effect of bronchodilator on biometrics, none of product[20]
Fundability Worksheet ScoresCompetitors Customer Validation IP Position Works Cited1. “Asthma Medications.” WebMD, WebMD, www.webmd.com/asthma/guide/asthma-medications#1. Accessed 30 Aug. 2017. 2. "Pneumoconiosis." Pneumoconiosis | Johns Hopkins Medicine Health Library. N.p., n.d. Web. 06 Sept. 2017. http://www.hopkinsmedicine.org/healthlibrary/conditions/respiratory_disorders/pneumoconiosis_134,162/ 3. “Health effects of coal.” Health effects of coal - SourceWatch, www.sourcewatch.org/index.php/Health_effects_of_coal. Accessed 5 Sept. 2017 4. “U.S. Energy Information Administration - EIA - Independent Statistics and Analysis.” China produces and consumes almost as much coal as the rest of the world combined - Today in Energy - U.S. Energy Information Administration (EIA), www.eia.gov/todayinenergy/detail.php?id=16271. Accessed 30 Aug. 2017. 5. U.S. Patent No. US4141703A. (1997). Washington, DC: U.S. Patent and Trademark Office. https://patentimages.storage.googleapis.com/dd/59/f4/faf5b1dc80a7e5/US4141703.pdf 6. U.S. Patent No. US7118608B2. (2004). Washington, DC: U.S. Patent and Trademark Office. https://patentimages.storage.googleapis.com/19/5c/a3/d17fee70eae4e6/US7118608.pdf 7. U.S. Patent No. US5701892A. (1995). Washington, DC: U.S. Patent and Trademark Office.https://patentimages.storage.googleapis.com/51/14/d5/d52d5b1d3a39b6/US5701892.pdf 8. U.S. Patent No. US6394088B1. (1999). Washington, DC: U.S. Patent and Trademark Office.https://patentimages.storage.googleapis.com/00/10/c5/76b9e74adb2163/US6394088.pdf 9. U.S. Patent No. US6394088B1. (1999). Washington, DC: U.S. Patent and Trademark Office. https://patentimages.storage.googleapis.com/6d/fa/01/0df16d874e8c0c/US6051566.pdf 10. U.S. Patent No. US6102036A. (1998). Washington, DC: U.S. Patent and Trademark Office. https://patentimages.storage.googleapis.com/04/d7/1c/53d6b83990da3b/US6102036.pdf 11. U.S. Patent No. EP0923957A1. (1998). Washington, DC: U.S. Patent and Trademark Office. https://patents.google.com/patent/EP0923957A1/en?q=inhaler&q=lung,A61M15%2f009 12. U.S. Patent No. US8037880B2. (2006). Washington, DC: U.S. Patent and Trademark Office. https://patentimages.storage.googleapis.com/6b/e6/73/9ce8ef5901fbb3/US8037880.pdf 13. U.S. Patent No. US20100008997A1. (2009). Washington, DC: U.S. Patent and Trademark Office. https://patentimages.storage.googleapis.com/15/2b/68/1791824bc600ee/US20100008997A1.pdf 14. U.S. Patent No. US20080276935A1. (2008). Washington, DC: U.S. Patent and Trademark Office. https://patentimages.storage.googleapis.com/e2/8c/4a/de094c76b9c119/US20080276935A1.pdf 15. U.S. Patent No. US20060228306A1. (2006). Washington, DC: U.S. Patent and Trademark Office. https://patentimages.storage.googleapis.com/cd/e8/51/81cd5724981dbf/US20060228306A1.pdf 16. U.S. Patent No. WO2005063732A1. (2004). Washington, DC: U.S. Patent and Trademark Office. https://patents.google.com/patent/WO2005063732A1/en?q=%22mast+cell+stabilizer%22&q=lung,asthma&page=1 17. U.S. Patent No. US5823180A. (1995). Washington, DC: U.S. Patent and Trademark Office. https://patentimages.storage.googleapis.com/29/84/02/38b12c5703297f/US5823180.pdf 18. U.S. Patent No. US08317407. (1994). Washington, DC: U.S. Patent and Trademark Office. https://patentimages.storage.googleapis.com/14/c2/86/c6869cf603d9c2/US5674860.pdf 19. U.S. Patent No. US20020076382A1. (2001). Washington, DC: U.S. Patent and Trademark Office. https://patentimages.storage.googleapis.com/c7/66/57/5ed36a149124a9/US20020076382A1.pdf 20. U.S. Patent No. US5340587A. (1989). Washington, DC: U.S. Patent and Trademark Office. https://patentimages.storage.googleapis.com/06/f8/1c/1a0296318ec85d/US5340587.pdf 21. Priddle, Robert. "Coal in the Energy Supply of China." International Energy Agency, Coal Industry Advisory Board, https://www.iea.org/ciab/papers/coalchina99.pdf. Accessed 5 Sept. 2017. 22. Yanfang, Wang. "Top 10 Hospitals in China." China, 11 July 2013, www.china.org.cn/top10/2013-07/11/content_29393416.htm. Accessed 5 Sept. 2017.
| |||||