BME100 f2017:Group1 W1030 L2
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | ||||||
OUR TEAM
LAB 2 WRITE-UP
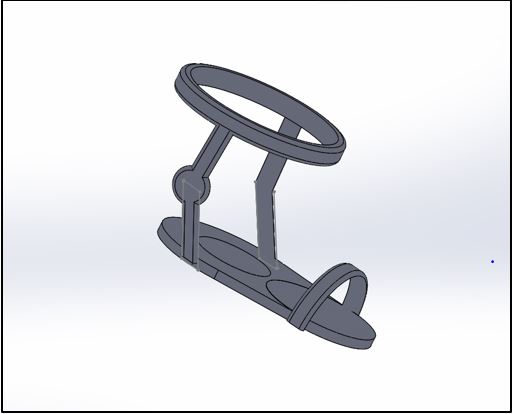
Description of Device This device will be capable of tracking different aspects of a customer's workout from the customer's foot. This device has several key parts, each working to provide the customer with numerical values that they can use to plan their routine workouts. First, the base of the device will be composed of a synthetic gel insole which covers the heel of your foot. Within, this insole will have a pressure sensor which could provide the user with a variety of data, including, but not limited to: weight of the user, steps taken and distance traveled. The second part of the device provides the support that the insole needs to stay attached to the users foot. This supporting structure will be composed of a couple of durable spandex bands that wrap around an individual's foot. One at the bridge of the foot, and the other at an individual's ankle. There will also be another band that runs from the ankle band to the bottom of the insole, making sure to pass through the posterior tibial artery. A sensor will be placed here on this artery in order to constantly measure the user's heart rate, which will give a more accurate reading of the number of calories burned. The entire device will be removable and machine washable. Also, the band that wraps around your ankle just below your calf muscle will serve as a marketing device as well. It will contain the name of the brand and the logo, and will come in a variety of different colors to maximize customization flexibility for the customer. The third and final part of the device will be another synthetic gel insole that attaches to the other foot. This device provides the same function as the insole in the other shoe; weighs the user, measures the amount of steps taken, and deduces the distance traveled. The reason for this second insole is to be able to more accurately measure each aspect that the insoles could measure, as well as distribute the weight between the two. All three parts of this device will be working together to provide data, which will be run through a software that will then interpret that data and provide some tangible statistics for the user. All of this data will be transferred wirelessly to a mobile device through bluetooth. The user can then download an app on their mobile device that will serve as the interface between the user and their collected data. Technical and Clinical FeasibilityTechnical Feasibility a. What are the technologies needed?
2.Pressure plate to collect the bulk of data for the consumer: tracking, weight measurements, and counting steps 3.Sensor to be fitted directly above the posterior tibial artery: measures heart rate consistently- whether the consumer is in motion or not- every 10 seconds [compare to Apple Watch- https://support.apple.com/en-us/HT204666] 4.One-size-fits-all design 5.Waterproof casing for sensor, sole and pressure plate 6.Recharging capabilities- at least 18 hours of battery life with maximum activity of the consumer
2.Continuous heart rate monitoring- can drain battery life and may not be as feasible 3.Battery that is small enough and able to retain at least 18 hours of battery life 4.Finding a pressure plate that can measure weight of the customer within a fairly accurate range- +/-20% weight (ideal for calorie counting, not for consumer use) 5.Ensuring that all sensors and aspects of the device can be made waterproof
2.Lack of ability to create the one-size-fits-all model 3.Failure to monitor heart rate consistently throughout the day 4.Inaccurate calorie counting- though our goal is to improve accuracy as opposed to other models
Clinical Feasibility
2.Patients at risk for obesity may find it encouraging to track their calorie intake and reduce their risk of becoming obese. 3.The device will be pleasing and small enough to be used as a type of medication or possibly as an additional requirement for health issues.
2.Patients may diagnose themselves with a disease based on the numbers they are seeing on the fitness tracker. 3.If any defects are present with regard to counting calories and translating the calculations to consumers, those trying to combat obesity may not make any progress- and will begin to lose confidence in the product 4.There is a possibility of creating an obsession with counting calories- rather than just portion control and eating the rights foods- which may create even more difficulty for patients
-The trial lasted a total of 12 weeks 2.Lancaster General Hospital in Pennsylvania began a trial in April of 2017 -Trial is ongoing 3.Children’s Hospital Boston will begin a trial in December of 2018 to increase activity for young cystic fibrosis patients. 4.University of California, San Francisco began a trial in December of 2016 to prevent diabetes in adolescents with Fitbit devices -Trial lasted 3 months 5. Cedars-Sinai Medical Center in Los Angeles is using Fitbits to evaluate cancer patients’ fitness for chemotherapy -Trial began in September of 2016 and lasted 2 weeks 6.University of Minnesota is using fitbit to evaluate the quality of life for brain tumor patients -Trial will begin in September of 2018 7.Duke University will begin a trial in December of 2017 that includes using Fitbit data in a tailored text message campaign to increase physical activity for cancer survivors -Trial will last 12 weeks 8. University of British Columbia in Vancouver will conduct a trial increasing physical activity in patients with arthritis using Fitbits in October 2017 -Trial will last 6 month 9.Duke University with Verizon completed a trial in August of 2016 which was a mobile health study in patients with peripheral Artery Disease -Trial lasted 28 weeks 10.Joslin Diabetes Center conducted a trial in March of 2017 which tested a digital diabetes education and management platform in patients with Type 2 Diabetes -Trial is still ongoing until December of 2017.
Market AnalysisValue Creation 1. Provides the customer with a more accurate measurement of bodily factors: weight, heart rate, steps taken, distance, calories burned; with steps taken and distance measurements being more accurate than the iPhone and Apple iWatch. 2. Creates peace of mind for customers dealing with obesity as they can have a constant tracking system of potential health dangers. 3. Comes in great use for athletes as they could keep track of important factors in their fitness and could help them to attain their fitness goals.
1. A pressure plate or Square Force-Sensitive Resistor (FSR) from Adafruit Industries costs $7.95. They are made of plastic and have a sensing region. While these plates can detect weight, they are not very good for determining how many pounds are on them. -(https://www.adafruit.com/product/1075?gclid=Cj0KCQjwruPNBRCKARIsAEYNXIg7qZDazVGXb0-hBABO1PbHExR_RTFTiikPzXZVoXqtMT-buwSFOg0aApG_EALw_wcB) a.Dimensions Length: 88mm/3.47 Width: 43.7mm/1.72in Thickness: 0.42mm/0.0165in Weight: 1.12g/0.04oz 2. Custom solutions from Flintec-> Force sensor MBC Through-Hole Force Sensor → estimates ($) based on how many units you buy (https://www.flintec.com/weight-sensors/force-sensors/miniature/mbc) High accuracy ± 0.25% Low profile and small diameter Range of internal thru-hole diameters Compression force measurement Low weight Stainless steel construction Temperature compensated -10°C – + 40°C Environmental protection to IP65 RoHS – Lead free approval 2. A roll of wholesale spandex costs $14.00 per yard(36’’x66’’). The bands we will make will be 1’’ by 6’’, making 11 bands per roll of spandex. That being said the price would be roughly $1.27 per band -http://www.spandexhouse.com/products.php?navId=139&navName=Solid%20Colors/Nylon 3. PowerStream technologies has developed rechargeable batteries that can range from .5-2mm thick. These batteries can last up to 2 years in stand-by mode and generate more than enough energy to power this product. For an individual battery it costs, depending on size, $6-$10. However when bought in bulk (5000+) it costs between $2-$3 -https://www.powerstream.com/thin-lithium-ion.htm 4.Heart Rate / Pulse Monitor from Arduino The highest priced one on ebay from China, costs anywhere from $1-$4 $25 from Adafruit Industries 5. Gel Heel Insoles from Walmart are $3.39 -https://www.google.com/aclk?sa=l&ai=DChcSEwiuyIDL7KLWAhWDgrMKHUi-CNUYABABGgJxbg&sig=AOD64_0kd-2ASMMPduGoAI0EgwePunko_A&ctype=5&q=&ved=0ahUKEwi1m_zK7KLWAhULy2MKHbnLDxIQwzwICA&adurl= 6..Bluetooth chip BT832 - Bluetooth Low Energy (BLE) 5 Module -https://gridconnect.com/bt832-bluetooth-ble-4-module.html?gdffi=3e9c4a34f3574e0abee6fe90e994ce82&gdfms=D5CF05FFA9D242A4A46EA6ED66AC8ACA&utm_source=google&utm_medium=CPC&utm_term=&utm_campaign=Shopping%20-%20Wireless%20Modules%20&mm_campaign=477cea803cb14c83d8a99b6c7d0cd349&keyword=&mkwid=sxbsCqZ2Z_dc%7Cpcrid%7C178600699322&gclid=Cj0KCQjwruPNBRCKARIsAEYNXIgH6UhtbkexdmKFfEbzn-Q_vgSFaEuC8althOiqnFAuGa5fmy_muBUaAnJgEALw_wcB costs is about $5.50 7. Labor Costs / Manufacturing Costs
When all the above components are added together, the ASP is $25.11- the sales price will be ~ $26.00.
US Population * 0.05* 0.36 * Price= 1.453*10^9
Fundability DiscussionScores Customer Validation → 1 Market Size → 3 Competition → 2 IP Position → 2 Technical Feasibility → 3 Explanation This device is fairly straightforward, with existing technologies that can be put together with ease to create value for the customer. Obstacles lie primarily in continuous heart rate monitoring while upholding the necessary battery life; this issue will be addressed during product development. Other challenges lie in creating a pressure plate that is compact enough to maintain the sleekness of the device while holding the capability to measure weight +/- 20% for calorie burning calculations. Despite these obstacles, it is very likely that this product can be developed successfully. Regulatory Pathway → 1 Clinical Feasibility → 2 Explanation The clinical feasibility of this device is not as strong as its technical feasibility; the reason for this exists in the potential for various clinical issues to arise during clinical trials/ implementation of the device: self-diagnosis based on reports of physiological processes that occur within the customer's body, a reversal in weight loss due to inaccurate reports of calorie calculation or false 'permission' for the customer to make bad lifestyle choices just because they are burning calories and the development of an obsession with calories which can lead to eating disorders. There are a number of possibilities which pose challenges to the ethical side of product development in this case. Clinical trials are extremely important- and while their implementation may be feasible, it is likely that it will take a significant amount of time to ensure that the customer's well-being is protected. Total Score → 72
|
||||||