BME100 f2017:Group14 W1030 L2
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | ||||||
OUR TEAM
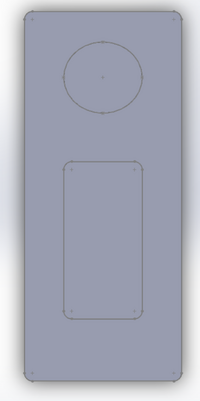
LAB 2 WRITE-UPDevice Image and DescriptionThe prototype consists of 3 different parts itself, the top the middle and the bottom sections. The top section is the area that the solution is added to and where the results are listed at. The middle section houses the "sheet" that the solution goes on to, and the bottom section acts as a point to hold the "sheet" in place so it does not come out, and it keeps the "sheet" clean until use.
Technical and Clinical FeasibilityTechnical Feasibility a. What are the challenges? Some of the possible challenges we will face include the technological aspects that test the urine. The chemical reaction that the Gonorrhea and Chlamydia test is based off of uses a swab instead of urine. Our group instead wants to be able to detect the bacteria in the urine rather than a swab. The test has to have a high accuracy rating. Another challenge is making the product easy and straightforward to operate. The package also must include easy to read directions that any individual could understand without difficulty. b. What could go wrong? The test needs to have proper packaging to prevent spillage and contamination. It is possible during shipping and handling that the buffers and tests could be damaged. The individual who is being tested will need to mix buffers and apply them to the test. It is possible for someone to accidentally mix incorrect buffers or incorrectly apply the solution to the testing stick. Clinical Feasibility a. Will it work in the clinic? Market AnalysisValue Creation 1) The prototype is cheap to manufacture, user-friendly in terms of size and operation. - Its small size helps it fit in almost any bag, purse etc. 2) The total cost to make the Chlamydia and Gonorrhea test is $30. The cost breakdown is demonstrated below in part b. -0. 05 N Sodium hydroxide solution Buffer A: 65 Cents per kit -Tris-HCI buffer pH 9.0 with 0.02 % sodium azide Buffer B: 80 cents per kit 3) A detailed instruction manual will be included with the product, so as long as the customer follows the instructions, they should have accurate results within 15 to 20 minutes. Since there are two tests in one box, the customer just has to pay attention to make sure that they keep the two tests separate. Additionally, the kit can be set up and used in any private space that you can occupy for roughly 15 minutes that has a flat surface that can be used to mix solutions. Since the test requires a few pieces (such as multiple test tubes, an eye dropper, and the test itself) we estimate the box to contain all of the merchandise to be about 7”x5.5”x5.2”. This packaging will be small enough to fit in a purse or medium sized bag.
Assuming we choose to use a cheap packaging, the box for the product will cost roughly 40 cents. The immunoassay strip and plastic housing will cost approximately $1.20. The buffers for the Chlamydia test will cost $1.55. The small plastic containers for the buffers will each cost 20 cents per container, giving a total cost of 80 cents. The pipettes will each cost 10 cents, and the mixing tubes will each be 20 cents. Sales Price Since the anticipated sale price should roughly be five times the cost to create the product, we estimate the sale price to be $30. ($5.00 additional as safety)
57,656,220
Fundability DiscussionUsing the fundability worksheet, I do not believe that our prototype would be funded. This is because we only received a score of 4. The majority of the rows received a rating of 1 or 2 because there is a similar product, but our product is fairly complicated and therefore would take a lot of testing to ensure that the product is accurate and produces the desired results.
|
||||||