BME100 f2016:Group8 W1030AM L2
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | |||||||
OUR TEAM
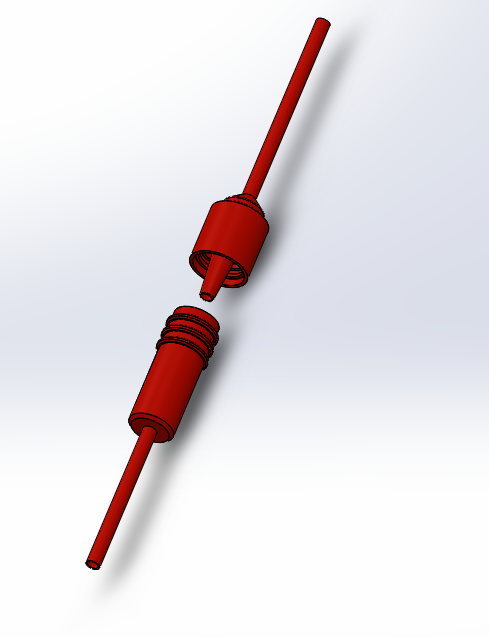
LAB 2 WRITE-UPDevice Image and Description
Technical and Clinical FeasibilityTechnical Feasibility a. The technologies needed to produce Luer connectors are widespread and are mostly manufacturing needs. These would include CNC machining and injection molding. The unique connectors would be engineered; molds machined with CNC lathes, and finally used in injection molding to rapidly manufacture thousands of parts. These would be sterilized, sealed and shipped to clinics and hospitals. b. The challenges include replacing hundreds of thousands of stock of the already ubiquitous Leur connectors and facilitating changes in procedure. There are few anticipated challenges with the actual engineering and manufacturing of a plastic connector, as it doesn’t present new technological processes. c. Potential risks include faulty connectors, leaks, breakage etc. It is imperative to test, iterate and recruit the best expertise in the fields of medical fittings, plastic engineering, manufacturing and sterilization. Given the proper engineering procedure, the product is expected to be successful.
Yes our product would work in a clinic because it isn't too different from products that physicians and nurses have already been trained to use. Our connector would be especially useful in a clinic because it has added safety features. Our connector will be used for vascular, eternal, epidural, respiratory, and intrathecal tubes in the clinic. If the connector or tube is not washed, there is a chance that bacteria can grow. A similar product has been tested before in a clinical trial. A needle less connector was tested for microbial contamination after repeated use. A three way stop clock luer with a standard cap was used and was compared against a Clearlink Y-Extension set. The results were recorded and compared against each other. A total of 60 patients were used, and were recorded for 72 hours each. Market AnalysisValue Creation Manufacturing Cost Sales Price Market Size
http://www.cdc.gov/nchs/fastats/emergency-department.htm Fundability DiscussionOur current scores from Competition, Customer Validation, and IP Position are 2, 2 and 3 respectively. In addition to that we would have to give ourselves a score of 0 for market size because our estimate of 55 million is well below the 80 million threshold. For Technical feasibility our product receives a 3, because the technology is relatively straight forward. For Clinical feasibility it receives a score of 3 as well because similar products are widespread throughout hospitals today. Unfortunately although our scores are good in all of the categories other than market size, the one zero brings our total score to zero. This would make our current product not fundable unless we can create enough value to to increase the price and therefore dramatically increase the market size.
|
|||||||