BME100 f2016:Group6 W8AM L2
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | ||||||
OUR TEAM
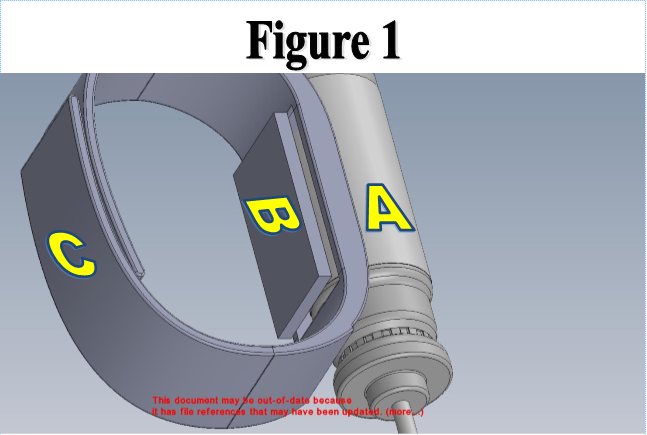
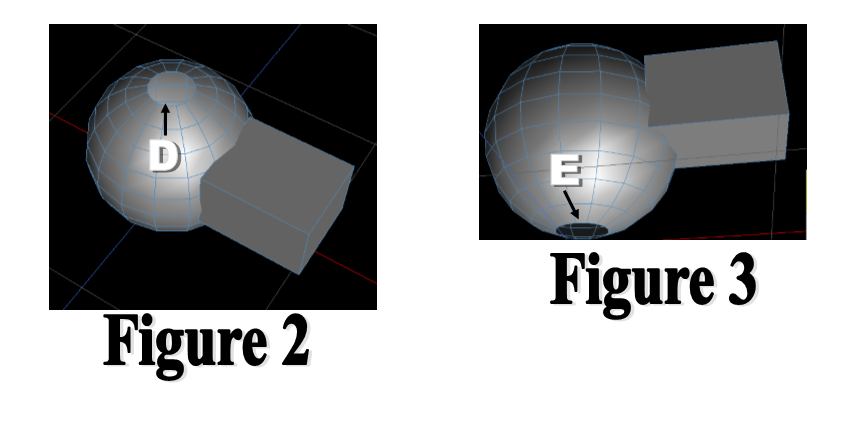
LAB 2 WRITE-UPDevice Image and DescriptionFigure 1 shows the inhaler wristband and blood oxygen monitor prototype. The part labeled 'A' is the inhaler canister, easily replaceable by the user. This will be encased fully in the silicone of the bracelet in a form-fitting manner, with openings at the top of the canister for replacement and at the bottom for use. The part labeled 'B' is the blood oxygen monitor, a small chip that will be able to register pulse and blood oxygen, as well as many other factors. Part 'C'is the adjustable part of the wristband, which will be held together using either a buckle or a plastic loop similar to those used on animal flea collars. Figures 2 and 3 are prototypes of the mouthpiece for the inhaler. This will be attached using silicone with a simple hinge for easy rotation. It will lie close against the wrist, using the hole labeled 'E' to keep the nozzle of the inhaler from accidentally discharging during daily activities. The hole labeled 'D' will be where the nozzle will engage for speedy use. The user will have to physically disengage and rotate the mouthpiece in order to discharge the inhalant.
Technical and Clinical FeasibilityTechnical Feasibility a) The face of the device would display the date, time and live pulse and bloody oxygen level readings. The face of the device would incorporate pulse oximeter technology in order to provide current and continuous readings. The monitor and device would be wireless and use Bluetooth to pair with the user's smartphone to display a history of readings, as well as the current readings. The app itself would be designed to better secure a pediatric market by giving parents a way to access their child's vitals while they are away or asleep. Additionally, a vibration monitor system will be incorporated into the face of the device to alert the patient and/or parent when the vitals drop below a designated level, determined individually with the assistance of a pulmonologist. b) The greatest challenge with the device is the technology needed to give live and constant pulse and blood oxygen level readings. The current technology enables a pulse oximeter to measure a single, current blood oxygen level when prompted. In order to fit the new device, new technology would be needed to allow a pulse oximeter to continuously measure and report blood oxygen levels. Additionally, blood oxygen levels are currently measured by readings taken by a device attached to the finger. Ideally, new technology would allow blood oxygen levels to be measured on an individual's wrist without the need of a finger attachment. c) Without the technology necessary to obtain a reading from the wrist, the device would be much bulkier and run a higher risk of breaking or not being used. The finger band needed to gather readings would need to be attached to the band itself in order for the readings to display on the face of the band, limiting movement and restricting the use of the hand in daily life. This could ultimately lead to either the destruction of the wire connection or the frustration of the patient, both resulting in the removal of the device. Following the development of the new technology necessary to obtain wrist readings, the device would run the risk of having pauses or breaks in current monitoring. During these freezes, a user could suffer low oxygen levels and the device would be unable to alert them. This risk would prevent the user from benefiting from the alert system in the device, but would still allow them to benefit from the convenience of their inhaler on their wrist.
a)This device would be very capable in a clinic because it is designed to be used for asthma prevention, as opposed to a possible cure. Children who suffer from chronic asthma can be monitored throughout the day using the device to send their vitals to a health physician, as well as their parents. The children would wear the device on their wrist similar to a watch. The software in the face of the watch would allow the device to connect the information gathered by the monitor to an app which would allow parents or doctors to monitor the child's vitals on their smartphones or computers. In the clinic we would monitor the accuracy of the vitals taken by the device and whether it can help the children have easier access to the attached inhaler. We would also be testing the compatibility of the app with any type of phone so that there are no risks for a lack of data or receiving it late. b)The device itself has no risks because it doesn't involve any new drugs and it is not invasive. One possible risk would be if a child took the wrong dose of their inhaler. Although the dosage taken by the child is not directly tied to the monitoring device, an incorrect reading could lead to the misuse of the drug, such as a higher dosage than necessary. Another risk would be a malfunction with the app resulting in data not being transferred correctly or in the time needed to perform treatment. However, the app is meant for prevention so the device would not be causing any additional health issues for the child. c)A clinical study would prove very successful because there have already been similar clinical trials using a similar device for asthma prevention. One clinical study proved to be successful in freeing activity limitation using a device called a Healthy Buddy which many of the children in the trial liked because it was fun and it helped remind them to take their medicine (Guendelman, P. S., 2002). The trial lasted 90 days and its main goal was to find out if keeping track of asthma symptoms could help the family and physicians better the child's asthma care. The children were taught how to use the device with proper techniques answering different questions by a nurse that could access their asthma symptoms.
Market AnalysisValue Creation Manufacturing Cost Inhaler portion: $30. Patients without insurance can expect to pay between $30 and $60 for their medication along with the inhaler. The cost varies depending upon the dosage and brand of medication. Regular silicone wristband plus silicone inhaler casing: $35. To insure that the inhaler and the medication canister are secure attached to the wristband, a silicone casing will be provided to prevent fallout and damage. This is expected to cost around $15. A simple yet sturdy silicone wristband conjugates the major portion of the device. It is expected to be completely flexible for added comfort, yet robust enough to carry a monitor and medication, costing around $20. Blood-Oxygen Monitor: $100. Although a bit ahead of its time, the main portion of the device, the blood-oxygen level monitor, is expected to cost around the same price as the "Withings Pulse O2" device. An added feature, along with the phone application, is expected to be an alarm altering the patient of low blood-oxygen level.
Market Size
Fundability DiscussionCustomer Validation Technical Feasibility SourcesAsthma Statistics | AAAAI. (n.d.). Retrieved September 21, 2016, from http://www.aaaai.org/about-aaaai/newsroom/asthma-statistics By Product – Dry Powder Inhaler, Metered Dose Inhaler, Nebulizer; By Technology – Manually Operated, Digitally Operated; By Disease Indication – Asthma, COPD, Pulmonary Arterial Hypertension. (n.d.) Guendelman, P. S. (2002). Improving Asthma Outcomes and Self-management Behaviors of Inner-city Children. Retrieved September 14, 2016, from http://archpedi.jamanetwork.com/article.aspx?articleid=191535 How Much Does an Albuterol Inhaler Cost? - CostHelper.com. (n.d.). Retrieved September 21, 2016. http://health.costhelper.com/albuterol-inhaler.html Sethi, M. (2014, July 3). Pavlok [SLDPRT]. Grabcad.com. T. (2012, November 29). Generic Inhaler [SLDPRT]. Grabcad.com.
|
||||||