BME100 f2016:Group3 W1030AM L3
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | |||||||||||||||||||||||||||||||||
|
OUR TEAM
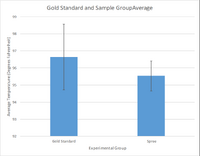
LAB 3 WRITE-UPDescriptive Stats and GraphTemperature
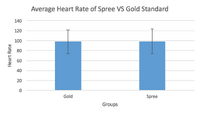
Heart Rate
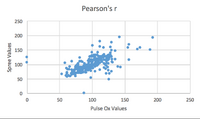
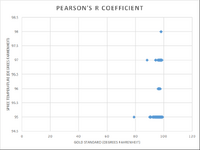
Inferential Stats
Design Flaws and RecommendationsOne issue with Spree is that it has a greater standard deviation value for measuring heart rate than the pulse-ox's measures. (The pulse-ox was the gold standard in this situation.) This means that the values measured by Spree differ more from the mean than the gold standard’s measurements. The standard error was also greater in the Spree headband than the gold standard. The standard error measures of the statistical accuracy of an estimate and it also takes into account the distribution of these measures. A large standard error value means that the values are spread apart and not very accurate. The makers of spree should invest in better sensors or in sensors similar to ones found in pulse-ox readers so the values of temperature and heart rate are more accurate (similar to the gold standard) as well as more precise (closer together/less deviated).
Experimental Design of Own DeviceThe subjects chosen for our study will be healthy individuals ranging from 18 to 30 years of age. In order to participate in a clinical study, one must be 18 or older. The reason why we capped the age at 30 is because a majority of the population that will be using our product are under the age of 30. Also, it is very common for individuals to outgrow their allergies in adulthood. The initial trial should include approximately 10 people because the initial trials are intended to examine if the device is safe; it is standard procedure for these safety trials to include approximately 10 subjects. The experiment will measure how epinephrine delivered by our implantable device compares to the epinephrine levels of subjects who had epinephrine delivered intramuscularly, like with an Epi Pen. The levels of adsorption of epinephrine into circulation of our implantable epinephrine delivery system will be measured and be compared to the gold standard’s (Epi Pen/ intramuscular injection). | |||||||||||||||||||||||||||||||||