BME100 f2016:Group1 W8AM L5
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
OUR TEAM
LAB 5 WRITE-UPPCR Reaction ReportTo start the experiment samples of DNA from two separate patients was provided, as well as a sample that was known as positive for PCR gene and one that was known to be negative for the PCR gene, and PRC reaction mix. A micropipette was used to transfer 50 uL of each sample of DNA from the patients as well as the positive and negative DNA samples into knew PCR reaction tubes. Each sample was put into a different tube, and a new pipette tip was used for each sample. 50 uL of the PCR reaction mix was then placed into each different tube, with a new pipette tip was used each time again to keep the samples pure.Visually it appeared as though all of the tubes had the same volume after the DNA samples and the PCR reaction mix had been added. The new reaction mixes were placed into the PCR machine which ran until the reaction was run to completion. When using a micropipette it is important to change the tip every time you work with a new sample and to set the desired volume on the pipette. The first stop verses the second stop of the pipette insures that the solution is not let out too quickly and also ensures that all of the solution is gotten out of the pipette tip. Fluorimeter Procedure
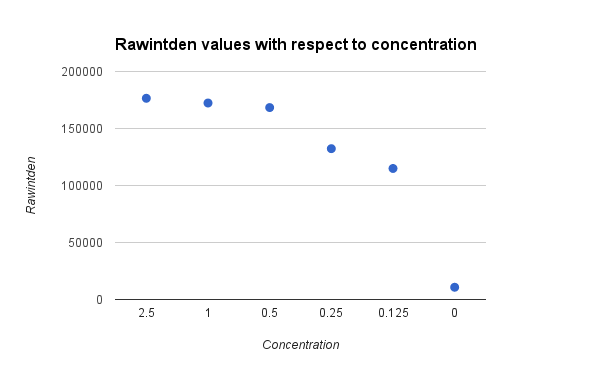
Data Collection and AnalysisImages of High, Low, and Zero Calf Thymus DNA
Images of Our PCR Negative and Positive Controls
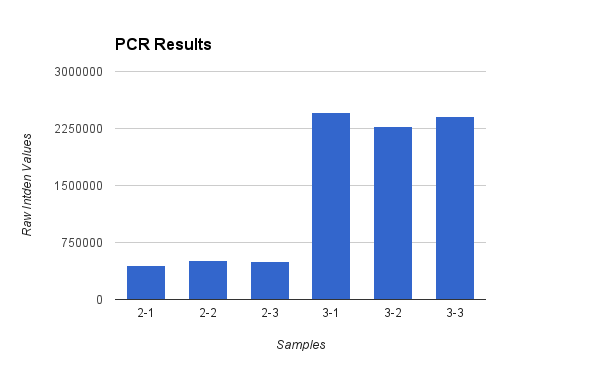
PCR Results: Summary
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||