BME100 f2016:Group1 W1030AM L2
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | ||||||
OUR TEAM
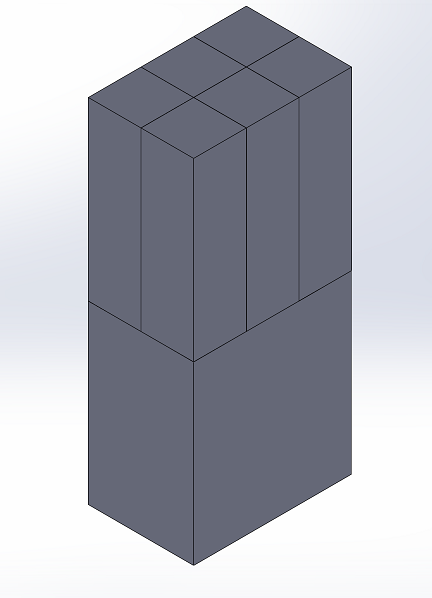
LAB 2 WRITE-UPLab 2: “Prototype Design: Part 1” o Leverage “Help” in SolidWorks and Custom video on Blackboard. Part 2 1. Design and draw your prototype in SolidWorks. Upload as an image and provide a brief description (including how addresses health issue) on Open Wet Ware. What value does your prototype create for the customer? 2. Determine the cost to create your design. Justify. 3. What would be the anticipated average sale price (ASP)? Justify. 4. Using initial market size analysis in Lab 1, determine the market size in dollars per year. 5. Using the fundability worksheet, determine if your prototype should be funded. Justify why or why not. Device Image and DescriptionTHE PILL POPPER The entire device is a rectangular box. Each interior pill dispensing box is 127 mm tall and 38 x 38 mm wide. The overall design includes 3 rows of 2 boxes for a total of 6 pill dispensing boxes in the entire device. The total width then is 114 mm and the depth is 76 mm. Inside the bottom base of the box is a ramp that connects to the inner edge of the top dispensing box. The ramp is 127 mm tall and 38 mm wide. The ramp extends diagonally 113.792 mm from each side of the box. The entire device is constructed from a durable metal compound. There are small sliding doors at the bottom of each pill box that have holes according to the size of the prescription pill. They will start closed and as they slide quickly across, the holes in the bottom will allow one pill to fall through. Each will pill will slide down the ramp once released from these holes. The next stage has another door that is locked by the biometric finger print sensor. This door will keep all of the time-released pills inside the unit until the fingerprint scanner has scanned the proper fingerprint. Then it will release all the pills. This device provides the customer with greater quality of life as they no longer need to keep close track of to take their medication. The "Pill Popper" will give the user more consistent, timely dosages without the worry of accidental overdose.
Technical FeasibilityTechnical Feasibility b. What are the challenges? The challenges are integrating the bio metric lock and the timer, figuring out how to dispense the pills, making the sensor send signals to the doctor/pharmacist, and designing the prototype in Solidworks. c. What could go wrong? The product could end up dispensing the wrong amount of pills. The doors could crush the pills if it closes too early. Mechanical and technical aspects could fail. The timer could fail. Batteries can fail. The doors could fail to unlock. The sensor could alarm the wrong person. Clinical FeasibilityClinical Feasibility a. Will it work in the clinic? Yes, of course. It is simple, easy to use by patients, caregivers and hospital coworkers as well. Pharmacists can easily learn the method of refilling and programming the pill dropper. Safety is a priority as well, multiple type of locks (one of the storage place, which can be only opened by trained pharmacists; one which allows the pills drop out onto a tray at a given time; one for the door of the tray, which can be opened with fingerprinting) secure the accessibility of medicines. Different pills are stored in distinct sections of the dispenser, not mixed up or contaminated by each other this way. b. What are the clinical risks? Accidental drop of more medicine or less medicine than necessary. People other than the patient having access to the medicine or the patient can't access them because of fingerprint scanning/door locking/opening/dropping error. c. Have similar products been in a clinical trial? How long was the trial? There are other devices on the market that are similar to our product. These products have been open to the public and available for purchase. Given that our product is a Class 1 device, it has minimum potential for harm, there should be no need for clinical trials, only manufacturer testing.
Market AnalysisValue CreationValue is being created here in multiple ways. General value created over the competition through various improvements. This device has a timer and a lock just like some other devices on the market. This device in particular though will allow for up to six different medications to be filled, and each one with its own possible timer settings. The timing for doses is put into the device by the pharmacist, and when it is time to take that medication, an alarm will sound. The person then must pass a biometric fingerprint scan to allow the medications to be taken at that time to fall down into the access door. There is the possibility of an alarm that would alert the pharmacy/pharmacist about people not taking their medication, and perhaps more importantly when the device is being tampered with, i.e. it could be set to send an alert if medication is dispensing (or one of the internal doors or devices allowing medication to dispense) at the improper time. This allows for closer monitoring of patients as to whether they are taking the medication at the right time, and also security for things like opioid prescriptions.
The insurance companies are who will pay the largest cost at the outset, but should see cost savings due to efficacy, eventually. The value then comes from adjusting insurance company costs--actually saving the insurance company money in the long run. (This could also turn into a feature of certain plans as a way to differentiate the competition.) Rather than the insurance companies paying for people to have treatment for opioid addictions (and probably often fighting about whether this should be covered and how much needs to be paid), this pill distribution device will prevent people from getting addicted in the first place. Data we found suggests that there were nearly 260 million prescriptions written for opioids alone, and that number is almost surely rising. The number of people addicted, needing treatment, or even dying, is also rising. Insurance companies are bearing the costs of that here in America. So, while there is an initial expense to get these devices for the consumer, it is a one time fee, at least until we find out how long they will last and need to be replaced, as in actually rebuilt. The insurance company will pay for (likely two) device(s) for the patient, but after that it is a small service fee each time the patient gets a new one with their new prescription, and returns the old one. This service/maintenance fee could certainly be passed on to the consumer, and would be considered small even by individuals.
Much of this value comes from the differentiation and improvements when compared to the competition. Value is created because they do not have to worry about a bunch of little pill bottles all the time, as six prescriptions can be loaded into the device, and if necessary, a second device could be obtained. Also, they do not need to worry about dosing information because, just as the pharmacist would fill regular pill bottles and put the dosing information on the label, they will load that information into the device, and the device will have time activated release locks, with biometric confirmation making sure that the right person is accessing the device and obtaining the pills at the right time. It is small enough to travel if necessary, and very safe with the biometric lock and tampering alert. Especially if insurance companies pay a part or large part of the initial device cost, there is no reason why a person who has any risks such as addicting medicine, kids or other relatives that might take pills, or who forget to take their pills, would choose not to use these, with a very small fee every month for cleaning and maintenance. And it beats carrying around six pill bottles and keeping the times and dosages straight in your head.
Pharmacies/pharmacists can know when patients are keeping the proper schedule for their medication. Also, more importantly, they could tell if someone is tampering with the device, and may be able to take action, including not prescribing those pills again without further measures. This will prevent misuse of medication, accidental overdoses, purposeful overuse, and accidental (or purposeful) use by someone other than the person who has the prescription. Another benefit that will come from the use of this product is for both the patient and the pharmacy/pharmacist. For prescriptions, whether dangerous/addicting or not, that are prescribed on an as needed basis, a fingerprint scan would still be necessary, and limits could be set, say no more than 2 pills in 6 hours, and this could be monitored for many reasons including abuse, efficacy, and improvement in the condition. Manufacturing CostPART 1 Biometric fingerprint reader and lock: Estimated price options: Version A: $50.00 Version A: The lock part of this door handle. Original current price: $82.59, this means we could get the lock from it only for about $50.00 probably. http://www.geekbuying.com/item/NEW-ADEL-LS911-Biometric-Fingerprint-Password-Door-Lock-L-R-Handle-S1---Black-337132.html?Currency=USD&catargetid=120190870000000238&cadevice=c&gclid=Cj0KEQjw6uO-BRDbzujwtuzAzfkBEiQAAnhJ0J7bGPeHTBf-ORgP6Q6LChJFkeASRocOOMx9MOLGAN0aAvln8P8HAQ Version B: $30.00 Version B: A fingerprint scanner connected to the device via USB connection. Original current price: $10.79, this means we could get it about $7.00 if ordered in large quantities. However, the use of this device could require an additional lock mechanism (estimated to be about a price of $10.00-$20.00). All in all, we know less about how the problem with this device could be solved, but the use of it seems to be cheaper. http://www.ebay.com/itm/Biometric-USB-Fingerprint-Reader-Security-Computer-Password-Lock-for-PC-QT-/161954835066?hash=item25b542aa7a:g:1iEAAOSwX~dWoYWD PART 2 Adjustable timer and lock for inside doors to drop pills onto tray at given times. Estimated price: $30.00 Timer lock of 'ksafe' ( http://www.thekitchensafe.com/ ), which is exactly the type our device needs. As ksafe is sold for $50.00 each. PART 3 Outside, strong safety lock defending the storage places where the pills are. This lock can only be opened by official pharmacy/hospital workers for cleaning and refilling, not by the patient or personal caregiver. Estimated price: Right now the goal is for the pharmacists to have access through the biometric lock, but their fingerprints (multiple pharmacists could be loaded into one device) would open the top of the device and allow for filling of prescriptions. This would be very similar to the device used for the internal door, except it would allow the outside top door to open. Not estimated to be a significant change in cost once the biometric lock is available to secure both doors. PART 4 Mechanism for outside and inside doors. Est. price: Somewhat rolled into the timer and biometric lock costs. Not estimated to be a significant change in costs once those two are in place. PART 5: Emergency alarms Est. p.: This type of technology is out there in things such as "Life Alert" which requires a much higher fee because there is constant monitoring and emergency contact available. We do not estimate this cost to be more than 10-15 dollars. PART 6 Framework, general material of device for sidewalls, doors and inner slides. Material: A cheap to find metal compound that is strong enough to resist common tampering. For example, resistance to a blow torch is not necessary because it would likely destroy the medications inside, and also would be extremely obvious upon return of the device. Est. p.: Hard to estimate for the group at this point, especially in the quantities we are talking about. PART 7 Electricity/energy resource. Est. p.: Cost of batteries. Summary: Estimated manufacturing cost: In the less than ideal case of expenses on the high end of these estimates, the device should not cost more than $150 to build. The mark up does not need to be excessive either as there are service fees every month, and probably two sold for each patient that needs one. Sales PriceSales price: (manufacturing price)*133%= $200.00 In order to make this device affordable to customers, pharmacies will purchase the devices and there will be a charge per month for the customer in order to cover any cleaning or damage fees in addition to the fee for refilling. The advised rental fee is $15.00/month but can be set by individual pharmacies. The Pill Popper will also be covered under some insurance plans. Shipping fees are not included in sales price. Some kind of guarantee for purchaser. Market SizeTarget population: Target population was divided into two according to market goals in different phases of device introduction. Phase one:
"In 2012, 259 million prescriptions were written for opioids, which is more than enough to give every American adult their own bottle of pills." http://www.asam.org/docs/default-source/advocacy/opioid-addiction-disease-facts-figures.pdf
Formulas: 1.(number of prescriptions)*(monthly rental fee in USD) 2.(number of annual patients)*(price of device in USD) Note: calculations with both formulas were done in order to show true market possibility, and as method of payment might be a subject to changes. Price of device was estimated to be about $200.00. Rental fee to be about $15.00/month. Phase one: For counting on opioid patients only: 1. 259,000,000*$15.00= 3,885,000,000 2. 21,583,333*$200.00= 4,316,666,600 Note: Because of the lack of relevant statistics, the number of annual patients was estimated by dividing the number of annual prescriptions by 12 (number of months in a year)(cc. 21 million).
1. 779,069,894*$15.00= 11,686,048,410 2. 64,922,491*$200.00= 12,984,498,200 Note: see sources for estimated patience and prescription number above at * sign. Summary - Market size
Of course these numbers assume 100% adoption. This would largely depend on whether insurance companies mandate adoption for prescription users. However, these can be scaled by any percentage of adopters, or by which insurance plans or prescriptions require the use of our product.
Fundability DiscussionCompetitors Customer Validation Clinical Feasibility Technical Feasibility Market Size IP Position Conservatively rated then: 1*1*3*3*3*1 = 27.
Regulatory Pathway Reimbursement Conservatively then, 27*9 gives a total score of 729, with three 1's that could be further evaluated. A working prototype would probably be a necessary next step, and given the market size would probably be worth investing in to get a rudimentary working device. Then it is highly advised to speak with insurance company reps and physicians/pharmacists to develop their interest, as well as patients to see their response to ease of use and safety, etc. This could potentially increase the values of Customer Validation and if they all see it as a positive, would likely increase the score for Competition as well, because the Pill Popper would be far removed from the competition at that point.
|
||||||