BME100 f2016:Group15 W8AM L5
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
OUR TEAM
LAB 5 WRITE-UPPCR Reaction ReportThe pipetting and PCR process was relatively smooth. We understood the that the difference between the first and second stop on the pipettor was that the first stop is used to absorb the liquid into the pipettor and the second stop is pressed to eject the liquid out. Our final reactions had the same amount of liquid as before the reaction. There was no liquid left in the DNA samples or PCR reaction mix. Our labeling scheme was consistent--it did not require any changes. Fluorimeter ProcedureImaging set-up First, we set up our smartphone camera vertically and placed it in the camera holder provided for us. We placed the slide onto the fluorimeter with the smooth side down. We then elevated the fluorimeter using several trays so that the camera was level with the slide on the fluorimeter. The camera was set to a timer of 5 seconds. After pipetting the fluorescent liquid and the solution we were measuring onto the slide, we took the picture and covered the phone and fluorimeter with a box so the picture could see the fluorescence more clearly.
Placing Samples onto the Fluorimeter It should first be noted that all of the drops must be placed on a fluorimeter slide. In order to ensure that proper bubbles are formed, the rough side of the slide should be the side facing upwards. Beginning with the SYBR GREEN 1 solution, we added 80 uL to the rough side of the slide. Following this, 80 uL of H2O were added right on top of the SYBR GREEN 1 solution. This gave us a clear bubble which we could analyze. The blue LED light was then turned on. Adjustments were made to make sure that the light went directly through the middle of the solution bubble. The box was lowered to cover the fluorimeter to allow for proper lighting, after which 3 separate images were taken of the solution bubble. This process was repeated for all of the Calf Thymus DNA concentrations, as well as the PCR DNA mix from the previous lab.
Data Collection and AnalysisImages of High, Low, and Zero Calf Thymus DNA
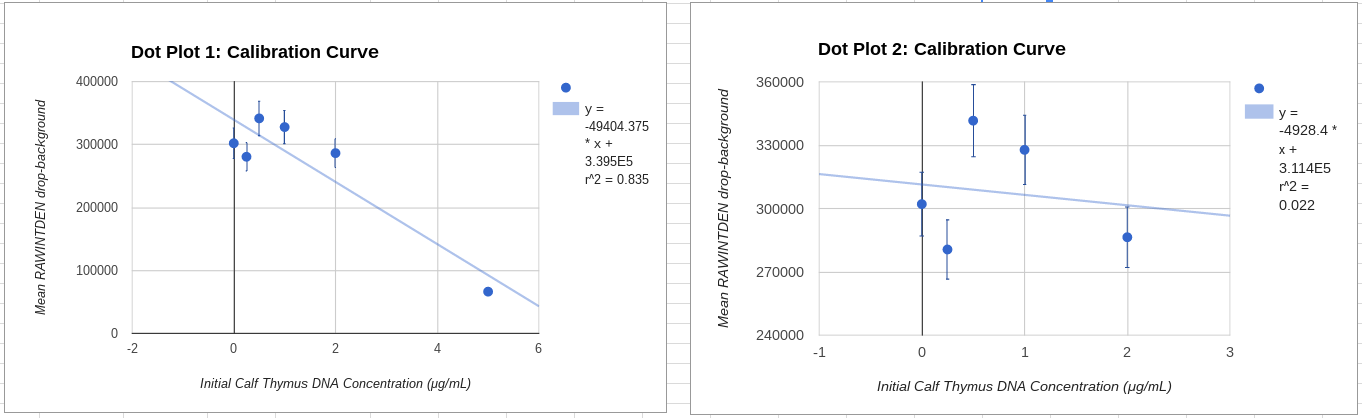
Calibration curves Images of Our PCR Negative and Positive Controls
PCR Results: PCR concentrations solved
PCR Results: Summary
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||