BME100 f2016:Group15 W8AM L2
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | ||||||
OUR TEAM
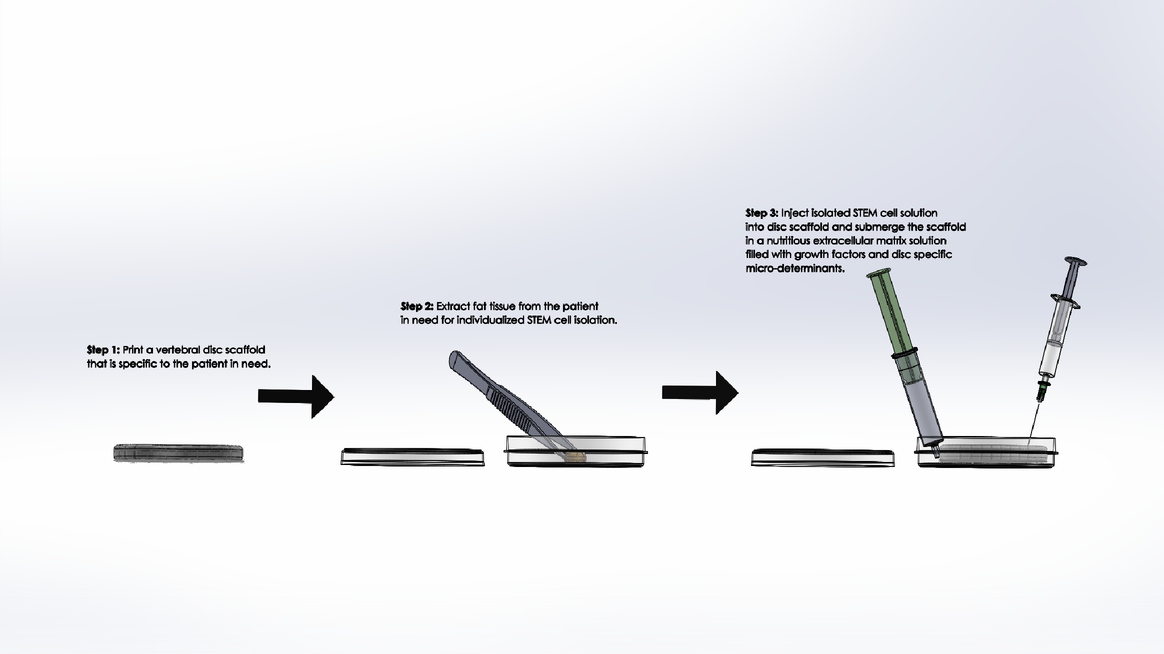
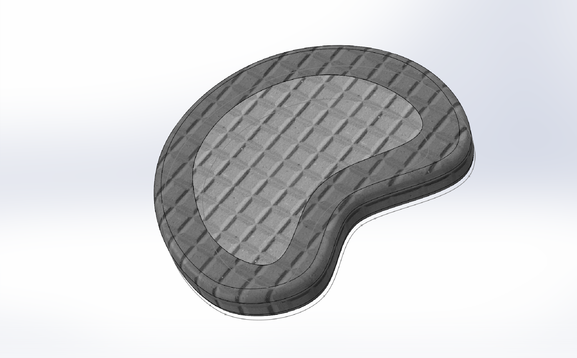
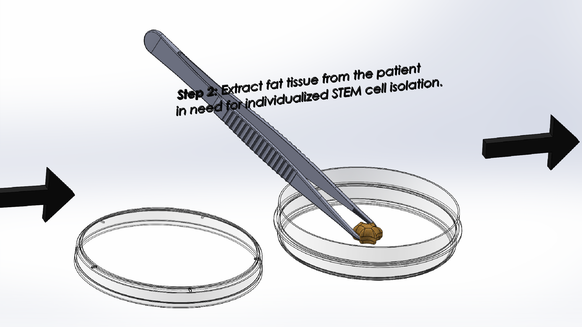
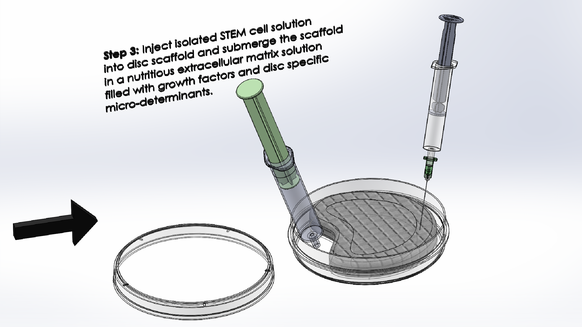
LAB 2 WRITE-UPDevice Image and DescriptionTissue engineering a vertebral disc may be the best solution to relieving patients with severe degenerative disc disease; however, it is not a simple process. It would be an expensive procedure that would involve multiple steps as outlined above. Because an engineered disc is meant to mirror a patient's naturally occurring discs, an MRI scan is required to prepare, with the expertise of a physician, an appropriate model of the disc to be 3D printed. After the model is conceived, as shown in step one above, a scaffold of the patient's new disc is to be printed with a fat-soluble polymer bio-material that will support the early disc cells until the disc is completely regenerated. After the scaffold is prepared, fat tissue is to be extracted from the patient and its STEM cell content is to be isolated in solution and cured in preparation for regeneration as outlined in step two above. Finally, the STEM cell solution is to be injected into the appropriate regions of the disc scaffold and the scaffold is to be submerged in a nutritious solution containing growth factors and disc-cell specific micro-determinants as described in step three above. The scaffold's surrounding solution will act as a critical extracellular matrix for the developing STEM cells that with give them the nutrients and chemical signals they need to become Anulus Fibrosis and Nucleus Pulposis cells. After a few weeks of submersion, the disc should begin to take shape. When the new disc is in a healthy enough condition to continue growth within the body, it is to be inserted in between the patient's vertebrae and the patient is to endure several weeks of recovery.
BDan. "Petri Dish 88mm Diameter." GrabCAD - CAD Library. GrabCAD, 18 June 2013. Web. 18 Sept. 2016. <https://grabcad.com/library/petri-dish-88mm-diameter-1>. Donnan, Jerry. GrabCAD - CAD Library. GrabCAD, 2 July 2011. Web. 18 Sept. 2016. <https://grabcad.com/library/5ml-syringe>. Dyakoff. "Tweezers." CrabCAD Community. GrabCAD, 19 May 2013. Web. 18 Sept. 2016. <https://grabcad.com/library/tweezers--3>. Racic, Stefan. "Needle with a Syringe." GrabCAD - CAD Library. GrabCAD, 17 Aug. 2016. Web. 18 Sept. 2016. <https://grabcad.com/library/needle-with-a-syringe-1>.
Technical and Clinical FeasibilityTechnical Feasibility
The technologies behind our product require various applications in emerging fields of science. A simplified version of the process is described below to illustrate the technologies required: First, an MRI scan will determine the dimensions of the patient's damaged vertebral disc. Using 3D printing technology, a scaffold will be created to match the proportions of the disc. It will probably be made of synthetic and polyester bio-material . Then, stem cells will be harvested from the fatty tissues in the arm and be given nutrients and other supplements to facilitate growth to fit the scaffold. When a healthy vertebral disc has regenerated, physicians will surgically implant the disc to replace the damaged one in the spine. The specific technologies that would be associated with this process include: MRI scanning, 3D bio-printing with synthetic polymer materials, STEM cell extraction and isolation, tissue engineering through regenerative medicine, and finally surgical equipment.
Considering how new many of the technologies and processes required for our product are, some of the possible challenges include the uncertainty of the application of these technologies. Regenerative medicine as a whole is a relatively new field in biomedical engineering that is still in development. Some challenges could include clinical issues concerning stem cells such as infection from poor sanitation in the laboratory or the possible rejection of the stem cells from the body. The body can reject transplants when the immune system does not recognize an object, and will attempt to destroy it. The nutrients involved, germs from laboratory, and scaffolding material, all present possible challenges to integrating the disc into the body (Engber). Other challenges arise over the utilization of 3-D printing technology to print the scaffold for the vertebral disc. The design of the scaffold must be exactly matched to the dimensions of the patient's disc, but also be malleable so that it can adapt to the shock-absorbent characteristics of a vertebral disc. Another possible challenge faced by patients is the longer waiting time in between requesting the surgery and performing the operation. Their disc must be cultured over time before the procedure can be performed.
Regenerative medicine through stem cell therapy could present risks of rejection from the body as well as control factors in the culturing of the disc in a lab. In addition to the challenges concerning rejection already mentioned, stem cells could pass on diseases and viruses from the nutrients involved in the growth process. Many of the nutrients used in current stem cell therapy require harvesting of cells from animals, which could carry harmful viruses or diseases (Murnaghan). This would increase the risk of the patient's body rejecting the transplant. Stem cells also present risks in the controlling of their growth. Stem cells can be volatile in the sense that they can grow very quickly and grow into many different kinds of cells (Murnaghan). Monitoring nutrient growth as well as its development into a vertebral disc will be critical in ensuring a healthy disc.
Several clinical trials concerned with implementing regenerative medicine solutions to degenerative disc disease (DDD) have been conducted in the past that show that our technology can work in the clinic. One recently conducted trial aimed to treat DDD with Allogenic Mesenchymal STEM cells began on May 18, 2016. Treating DDD with Allogenic Mesenchymal STEM cells is similar to our approach in using regenerative medicine to grow new vertebral discs from patients fast cells. The trial also measured two primary outcomes with the help of participants' responses to surveys collected for the duration of a year: safety and toleration as well as the pain and disability evolution (Treatment). This specific clinical trial validates the feasibility of regenerative medicine in clinical treatments of degenerative disc disease. However, it does not prove that tissue engineering a disc from scratch is feasible in the clinic. Since the technology to engineer a disc from scratch is still in development, whether or not it will work in the clinic is dependent on funds, research potential, equipment feasibility , and experimental success.
Replacing the vertebral disc is a complex operation, since it does require the physician to start from the stomach and work past the organs to reach the spine (anterior approach). Because the surgery begins from the anterior side of the body, it will likely be more invasive and thus need a longer recovery time compared to disc fusion, currently the most common treatment for degenerative disc disease. Another risk that regenerative medicine might go through is that the body of the patients may reject the STEM cells. Following the operation, there are many complications that could happen, such as infections, loss of blood, and since the physician will be approaching the anterior we pose the risk of hurting any organs in the process. Another risk is that the STEM cells may failure to mimic the properties of the original vertebral disc.
There have been two clinical trials that are that dealt specifically with fixing damaged discs with STEM cell regenerative techniques. One clinical trial is the above mentioned use of Allogenic Mesenchymal stem cells, and the other evaluates the safety of one single STEM cell solution injection injection using Rexlemestrocel-L Alone or combined With Hyaluronic Acid. Both of these studies are still ongoing and have no final results at this time. The treatment towards degenerative disc disease with Allogenic Mesenchymal stem cells started in May 2013. The second trial using one injection of Rexlemestrocel-L alone or combined with Hyaluronic Acid has been ongoing since March 2015 (Garcia). Another trial that started on October 2014, is using bone marrow from the sides of the hip bones to make bone grafts grown in the lab. The study looks at taking teaspoons from both hip bones (if necessary) during surgery in order to then place bone marrow in a machine for about 15 min that compresses the matter to a low volume of bone marrow aspirate concentrate, also known as BMAC. The BMAC is then combined with allograft bone chips using another machine to produce constructed bone logs that are placed along the backside of the spine (Passias). This specific clinical trial demonstrates what a practical procedure to artificially engineer a disc with the patient's own tissue and the help of an automated machine would look like when the technology is available. In combination, both clinical trials described above demonstrate the potential an artificially regenerated disc solution has in practice.
Garcia-Sancho, Javier, David Noriega C., Ana Sanchez 18, and Francisco Ardura. "Treatment of Degenerative Disc Disease With Allogenic Mesenchymal Stem Cells (MSV) (Disc_allo)." Treatment of Degenerative Disc Disease With Allogenic Mesenchymal Stem Cells (MSV). Red De Terapia Celular, 18 May 2013. Web. 21 Sept. 2016. <https://clinicaltrials.gov/ct2/show/NCT01860417?term=regenerative%2Bdisc%2Busing%2BSTEM%2Bcells&rank=1>. Murnaghan, Ian. "Concerns About Stem Cells." Concerns About Stem Cells. Explore Stem Cells, 9 Mar. 2016. Web. 14 Sept. 2016. <http://www.explorestemcells.co.uk/concernsaboutstemcells.html>. Engber, Daniel. "Why Transplanted Body Parts Get Rejected." Slate Magazine. Slate, 19 Jan. 2006. Web. 20 Sept. 2016. <http://www.slate.com/articles/news_and_politics/explainer/2006/01/tissue_rejection_101.html>. Passias, Peter. "Prospective Study of Thoracolumbar Spinal Fusion Graft (BMAC)." Prospective Study of Thoracolumbar Spinal Fusion Graft. New York University School of Medicine, 02 Oct. 2014. Web. 21 Sept. 2016. <https://clinicaltrials.gov/ct2/show/study/NCT02297256?term=body%2Bcells%2Bfor%2Btreating%2BDegenerative%2BDisc%2BDisease&rank=1>.
Market AnalysisValue Creation
Manufacturing Cost
65 million*$53,1257= $3.45326 trillion The product scores a 3 for market size on the fundability worksheet. The market size far exceeds the $500 million when considering the price of our product and the prevalent need for DDD treatment. The market for DDD treatments has high demand for a large population of patients. One reason, among numerous others, the demand is so high and we will continue to stay high is because of the high number of elderly patients in which DDD occurs naturally because of disc erosion over time.
Hunsberger, Joshua, Josh Neubert, Jason Wertheim, Julie Allickson, and Anthony Atala. "Bioengineering Priorities on a Path to Ending Organ Shortage." SpringerLink. SpringerNature, 1 June 2016. Web. 20 Sept. 2016. <http://link.springer.com/article/10.1007/s40778-016-0038-4/fulltext.html>. Knoepfler, Paul. "How Much Do Stem Cell Treatments Really Cost? - The Niche." The Niche. Paul Knoepfler, 05 May 2016. Web. 20 Sept. 2016. <http://www.ipscell.com/2015/02/stemcelltreatmentcost/>. Hochschuler, Stephen. "Issues to Consider Before Having Artificial Disc Surgery." Spine Health. Veritas Health, n.d. Web. 20 Sept. 2016. <http://www.spine-health.com/treatment/artificial-disc-replacement/issues-consider-having-artificial-disc-surgery>. Fay, Bill. "How Much A Doctor Visit Will Costs You - Blue Book Prices." Debt.org News. Debt.org, n.d. Web. 20 Sept. 2016. <https://www.debt.org/medical/doctor-visit-costs/>. Glover, Lacie. "Why Does an MRI Cost So Darn Much?" Time. Time, 16 July 2014. Web. 20 Sept. 2016. <http://time.com/money/2995166/why-does-mri-cost-so-much/>. Rodriguez, Charlotte. "Fact Sheet." INFUSE® Bone Graft. Medtronic, 21 June 2011. Web. 20 Sept. 2016. <http://wwwp.medtronic.com/Newsroom/LinkedItemDetails.do?itemId=1169645431867&itemType=fact_sheet&lang=en_IN>.
Fundability DiscussionClinical Feasibility Technical Feasibility Market Size Fundability
|
||||||