BME100 f2016:Group15 W8AM L1
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | ||||||
|
OUR TEAM
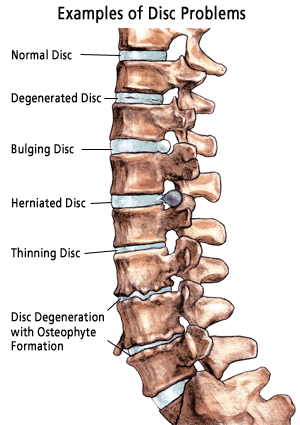
LAB 1 WRITE-UPHealth Care IssueDegenerative Disc Disease (DDD) is better considered an aging process rather than a disease because of how many people the condition affects on a regular basis. The vast majority of lower back pain (LBP) or neck pain cases are diagnosed as instances of DDD. The clinical need for effective cures and solutions to DDD is immense: The Health Quality Ontario Institute claims that 40% of all people aged 40 suffer from DDD and 80% of all people above the age of 80 suffer as well. Moreover, only 20% of these victims have a mild-enough condition that can be treated therapeutically and without surgery (Health 1). DDD is the term used to describe the degeneration of one or more intervertebral discs. It is the leading cause of neck pain, back pain, motor debilitation, and spinal cord irritation. A wide range of factors can cause a patient to develop DDD. The three most prevalent causes are traumatic compression injuries, old age, and heredity. Looking at the anatomy of the vertebral disc depicted below in figure 1, there are two important parts of the disc that deteriorate in cases of DDD: the Anulus fibrosus and the Nucleus pulposus. The Nucleus pulposus is the hydrated core of the disc that functions like a shock absorbing fluid similar to what is found in mechanical hydraulic system. The Anulus fibrosus is the fibrous tissue that surrounds the core and keeps it intact; this tissue is also stretchable and compressible. In all cases of DDD, one or more discs have diminished compression support for the two adjacent vertebrae due to a non intact Nucleus pulposus. This occurs when either the core has been dehydrated (usually due to old age or a hereditary condition) or the Anulus fibrosus has been badly damaged causing an unstable and weaken core (usually due to a traumatic injury). Figure 1: A diagram from the eHealthStar institute describing the anatomy of the Intervertebral Disc (Modric 1) Medical institutions like the Atlanta Brain and Spine Care Clinic have dubbed specific names to the various conditions that fall under DDD (refer to figure 2). A basic degenerated disc involves the accumulation of scar tissue on the Anulus fibrosus caused by repetitive minor injuries to the spine. A bulging disc involves the stretching of the Anulus fibrosus to a point where it makes contact with the spinal cord and/or surrounding nerves. A herniated disc involves the complete rupture of the Anulus fibrosus and the scattering of the inner core fluid tissue throughout the spinal cord's surrounding area, which is caused by a serious fall or other traumatic shock to the spine. A thinning disc involves the dehydration of the Nucleus pulposus, which is linked to old age and heredity. Lastly, degeneration with Osteophyte Formation is a condition where the spine adjusts to weakened thinning discs by building bone mass around the vertebrae to provide the spine with more support, which also causes great pain as bone gradually grown in the area surrounding the spinal cord (Benglis). All of these differing conditions have the same solution--vertebral disc repair. Figure 2: A diagram from the Atlanta Brain and Spine Care Clinic depicting various instances of disc degeneration that would be diagnosed as DDD (Benglis)
Works Cited Benglis, David M., Dr, and Roger H. Frankel, Dr. "Home." Lumbar Degenerative Disc Disease. Atlanta Brain and Spine Care, 2003. Web. 01 Sept. 2016. <http://www.atlantabrainandspine.com/subject.php?pn=lumbar-degenerative-disc-002>. Health Quality Ontario. “Artificial Discs for Lumbar and Cervical Degenerative Disc Disease Update: An Evidence-Based Analysis.” Ontario Health Technology Assessment Series 6.10 (2006): 1–98. Print. Modric, Jan. "Bulging, Herniated Disc and Pinched Nerve in Neck and Lower Back." Evidence Based Health Articles. EHealthStar, 17 Dec. 2015. Web. 01 Sept. 2016. <http://www.ehealthstar.com/conditions/bulging-herniated-disc>.
CompetitorsFusion, grafting of two or more vertebrae together to replace damaged vertebral discs, is currently the leading competitor in correcting degenerative disc disease (DDD) (Lee). This method has shown the most effective long-term results over other competitors such as disectomy/microdisectomy, arthroplasty and hybrid techniques (mechanical and hybrid implants), and non surgical methods such as physical therapy, physical supports, exercise, or pain medications. However, even fusion has many negative effects, such as limiting mobility in patients and increasing the risk of needing more surgery after the initial operation. The other methods are either not sustainable or simply prolong the time before corrective surgery is required.
Advantages
Disadvantages
Works Cited Lee, YC and Zotti MG, Osti OL. "Operative Management of Lumbar Degenerative Disc Disease." Asian Spine Journal, vol. 4, pp. 801-19. http://www-ncbi-nlm-nih-gov.ezproxy1.lib.asu.edu/pubmed/27548580 http://www-ncbi-nlm-nih-gov.ezproxy1.lib.asu.edu/pubmed/27559465. Accessed August 31, 2016 Nordqvist, Christian. "What is Degenerative Disc Disease?" Medical News Today, 26 Sep 2014. http://www.medicalnewstoday.com/articles/266630.php Subach, Bryan. "Breakthrough Cell-Therapy for Degenerative Disc Disease Sufferers." Virginia Spine Institute: Spine MD, 3 Oct 2013. http://www.spinemd.com/news-philanthropy/breakthrough-therapy-shows-promise
Customer ValidationPatient
Works Cited Health Quality Onario. "Artificial Discs for Lumbar and Cervical Degenerative Disc Disease-Update: An Evidence-based Analysis." Ontario Health Technology Assessment Series 6.10 (2006): 1-98. Print. "Insurance Covering Total Disk Replacement ? - Health Insurance Issues." Spine Health. N.p., n.d. Web. 02 Sept. 2016. IP Position=Patent Landscape: Currently, there are various methods and patents for treating Degenerative Disc Disease, repairing intervertebral discs, and even completely replacing them. Almost all of the latest patents involve the regeneration of the intervertebral discs through the use of biological material. A patent has already been filed and granted for the repairing of intervertebral discs by introducing stem cells to the already existing intervertebral disc. This patent is considerably the closest to our method. What separates our method from this one though is that we aim not to introduce stem cells to an already existing intervertebral disc, but rather to produce a completely new intervertebral disc from adipose tissue stem cells, coupled with a scaffold that will allow for the continuing growth and strength of this intervertebral disc. Using this newly made disc and scaffold, the damaged intervertebral disc can be removed and replaced with a brand new artificial one. IP Risks: As this method introduces an entirely new disc into the body, the biggest risk we face is getting the method and product approved by the FDA.
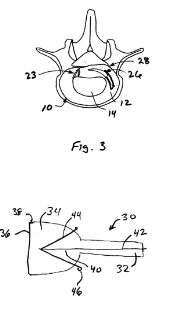
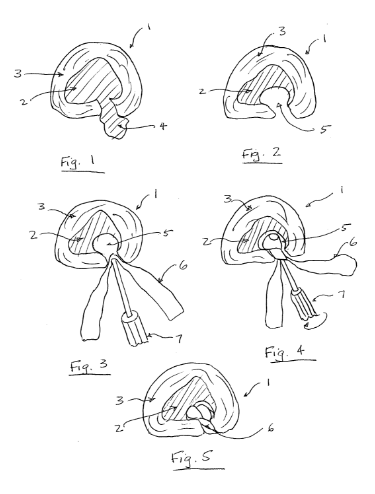
1. Patent #US20040193274A1
2. Patent #US20080045949A1
3. Patent #US20050149046A1
4. Patent #US20050069571A1
1. Trieu, Hai, Trieu Hai H., and SDGI Holdings Inc. "Materials and Methods for Augmenting And/or Repairing Intervertebral Discs." US20040193274A1. US Government, 30 Sept. 2004. Web. 02 Sept. 2016. 2.Hunt, Margaret, David Hooper, Alain Meunier, James Jara-Almonte, Hunt Margaret M, Hooper David M, Abbott Laboratories, and Zimmer Spine Austin Inc. "Method of Treating Degenerative Spinal Disorders." US20080045949A1. US Government, 21 Feb. 2008. Web. 02 Sept. 2016. 3.Friedman, Craig, Arindam Datta, and Friedman Craig D. "Repair of Spinal Annular Defects and Annulo-nucleoplasty Regeneration." US20050149046A1. US Government, 7 July 2005. Web. 02 Sept. 2016. 4.Slivka, Michael, Hassan Serhan, and DePuy Spine Inc. "Method for Treatment of Defects in the Intervertebral Disc." US20050069571A1. US Government, 31 Mar. 2005. Web. 02 Sept. 2016.
Fundability Worksheet ScoresCompetitors Customer Validation IP Position
| ||||||