BME100 f2016:Group12 W1030AM L3
| Home People Lab Write-Up 1 | Lab Write-Up 2 | Lab Write-Up 3 Lab Write-Up 4 | Lab Write-Up 5 | Lab Write-Up 6 Course Logistics For Instructors Photos Wiki Editing Help | |||||||
|
OUR TEAM
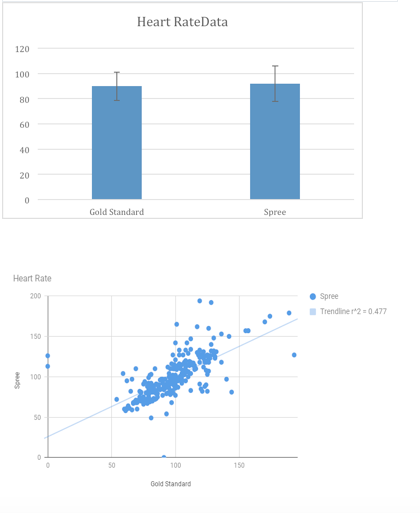
LAB 3 WRITE-UPDescriptive Stats and GraphHeart Rate:
*indicates significant difference between two values
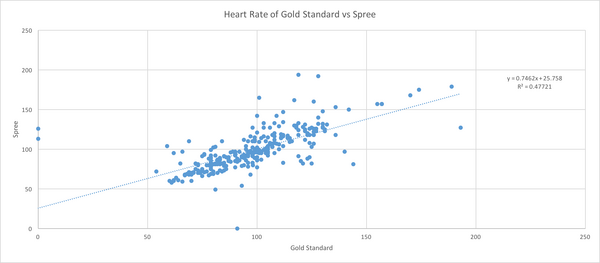
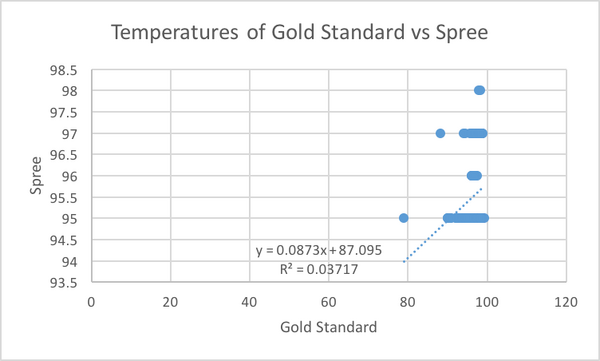
Inferential StatsHeart Rate T-Test: 0.427116193 Pearson's r for heart rate is closer to 1 than it is to 0, therefore there is a positive correlation between the gold standard and spree tests. If the value of the t-test is below 0.05 that means that there is 95% confidence level that a difference exists between the two values. The t-test value if above 0.05 which means that it is not certain that there is a difference between the gold standard and spree. For the spree testing, above a 0.05 was desired because we wanted the values to be as close as they could be. Design Flaws and RecommendationsDesign Flaws Compared to the gold standard, the spree headband records its temperatures in a way that is not as specific as the gold standard. There appears to be no correlation between the temperatures taken by the Spree headband, and the gold standard. The Spree headband records its temperatures in very generic ways, only able to list them in whole numbers such as 95, 97, etc., while the gold standard is able to record the temperatures of the wearer with very high accuracy, ranging from 94.2 to 97.86. Another flaw in the temperature recording is that the forehead is an unreliable location to measure temperature. When recording the heart rate of the wearer, there was a slight, positive correlation between the Spree headband and the gold standard. There do not seem to be any design flaws in the Spree headband that would cause this correlation.
Experimental Design of Own DeviceOur glucose monitor will require testing with 100 randomly selected type 2 diabetes patients. The participants will receive a blood test glucose monitor for the first part of the experiment to get baseline numbers. For the second part of the experiment, the group will receive our glucose monitor to see if our data is accurate. During both parts of the experiment, participants will be required to lead the same lifestyle, diet, and exercise that they would normally have, but make similar lifestyle choices from the first part to the second part. Inclusion Requirements: 18 to 75 years of age. Sign a consent form prior to clinical trials. Type 2 diabetes diagnosed 12 months prior to trial. Exclusion Criteria: Already participating in another study. Any disease which may inhibit compliance to directions for the study. Any chronic disease which may inhibit accurate readings. Chronic skin problems located on the wrist. Pregnant or breast-feeding women. Independent Variable: Blood test glucose monitor. Blood glucose levels of blood test monitor. Dependent Variable: Laser sensing glucose monitor. Glucose levels of laser sensor monitor. Predicted Outcome: If the design for our product is correct, then the values we get for the laser sensing blood monitor should be similar, if not equal, to those of the blood test glucose monitor. Reasoning: Taking a large group of 100 individuals allows us to account for any variance that each individual may have in their glucose levels. In order to ensure that the laser sensor is accurate, we will compare those results with those taken from the gold standard, a blood glucose monitor. We will test the device under the conditions of a normal lifestyle for each patient. This is done to ensure that the laser monitor can be used under normal conditions and will give the same accuracy that the blood test monitor would under the same conditions. The inclusion and exclusion criteria are set in place to ensure that the data can cover a large range of people while excluding those with conditions which may affect the accuracy of measurements that either glucose monitor may take. Finally, the independent variables are the blood test monitor and results while the dependent variables are the laser monitor and results. We will use the blood test as a control to which we can compare the laser monitor results. | |||||||