BIO254:Recycle
INTRODUCTION
Synaptic vesicles are neurotransmitter-containing, membrane-bound organelles. They are initially generated in the cell bodies of neurons, and are subsequently transported along microtubules to axonal terminals. Depolarization of presynaptic nerve terminals leads to CALCIUM INFLUX into the cell. The calcium influx triggers the vesicles to fuse with the plasma membrane and release their neurotransmitter into the synaptic cleft. The rate at which vesicles can be transported from the soma to axon terminals is not sufficient for continuous neural activity. This lead to the hypothesis of SYNAPTIC VESICLE RECYCLING.
Ca++ Influx & Vesicle Fusion
Synaptic vesicles filled with the appropriate neurotransmitters dock at the active zone of the pre-synaptic terminal in preparation for neurotransmitter release. When an action potential arrives at the nerve terminal, voltage-gated calcium (Ca++) channels open, causing a sudden influx of Ca++ ions. This pulse of intracellular Ca++ results in membrane fusion between the pre-synaptic terminal and release-ready vesicles. Two distinct components of neurotransmitter release are caused by the Ca++ influx: 1) A fast phase that is induced rapidly, in approximately 50 microseconds following intracellular calcium buildup, and 2) A slower component that continues for >1 s after an action potential (Barrett & Stevens, 1972). Both parts are dependent on the Ca++ influx.
Although fusion is triggered by the Ca++ influx, synapses do not remain static. Electrophysiological recordings of synapses by Katz et al. (8) demonstrated that synapses actually have a low probability of release. These rare vesicle fusions are spontaneous events of exocytosis that result in miniature postsynaptic currents.
Hallmark advances in our study of neurotransmitter release and vesicle recycling are made using the synapse formed by the calyx of Held (a giant brainstem terminal utilized for sound localization). This is the only synaptic model available that accurately accounts for all properties of vesicle fusion and neurotransmitter release (Meinrenken et al., 9). As its name implies, the calyx of Held envelopes the soma of a postsynaptic neuron like a cup, forming a large nerve terminal appropriate for study. About 500-600 synaptic contacts are made between the calyx terminal and the postsnaptic cell. Due to its size, it is possible to make direct electrophysiological recordings within the calyx terminal to monitor both pre- and postsynaptic processes simultaneously.
A crucial question about the synaptic vesicle pathway is how Ca++ channels are organized with respect to the vesicles. Using the calyx model, Borst and Sakmann (7) applied the buffer EGTA to calcium channels, but vesicle release was only inhibited by 50%. Their results suggest that the majority of vesicles are not directly linked to Ca++ channels, and that multiple Ca++ channels contribute to the total ion influx triggered by an action potential.
Synaptic Vesicle Recycling
Bittner and Kennedy initially proposed the idea of vesicle recycling in 1970 (1). Since then, multiple mechanisms have been demonstrated to take place during recycling. In 1973, Heuser and Reese demonstrated the first method of recycling at nerve terminals, called full-collapse fusion (2). In this method, the synaptic vesicles are completely flattened/incorporated into the plasma membrane. Another method, suggested by Bruno Ceccarelli in 1973, was later termed the “kiss-and-run” method. During kiss-and run, the synaptic vesicles fuse transiently with the terminal membrane and reform into functional vesicles (3).
Full-Collapse Fusion
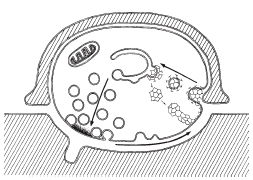
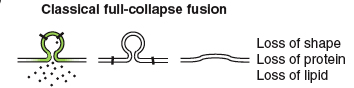
This method of fusion/recycling results in a nearly instantaneous release of neurotransmitter, followed by clathrin-mediated recovery of vesicle membrane. Using electron microscopy (EM) to visualize isolated frog neuromuscular junctions pre and post nerve stimulation, Heuser and Reese showed that depletion of synaptic vesicles following nerve stimulation correlated with an increase in plasma membrane surface area. Prolonged stimulation resulted in appearance of endosomes (cisternae) within the cell, which disappeared after rest as vesicles reappeared in the nerve terminals. To determine the timing of movement through these stages of vesicular recycling, they filled the synaptic cleft with horseradish peroxidase (HRP) and again using EM, looked at HRP localization after nerve stimulation. They found HRP in endocytosed coated vesicles immediately after nerve stimulation, followed by localization to endosomes and finally, an hour after stimulation, in synaptic vesicles. Due to the role of clathrin in this process, the recycling is inherently slow due to the kinetics of clathrin assembly/disassembly ( which, at fastest, are >30 s) (10). Although this method was originally observed in frog neuromuscular junctions, it has since been observed in mammalian central nervous system terminals.
Kiss-and-Run Method
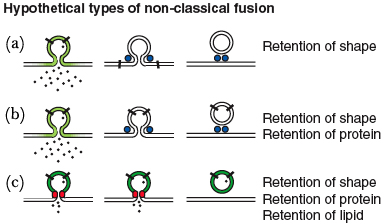
The kiss-and-run hypothesis states that neurotransmitter is released through the transient formation of a fusion pore between the vesicle and cell membrane. This term is used to describe various non-classical methods of fusion/recycling, but to date does not have a universally accepted definition (10). The first direct evidence for the kiss-and-run hypothesis was provided independently by Aravanis, Pyle and Tsien and by Gandhi and Stevens in 2003 (4,5). Both groups used fluorescent markers to track individual vesicles in hippocampal neurons following an electrical stimulation.
Aravanis et al used FM-dye labeling to study single vesicular events in hippocampal neurons. Cultures were bathed in FM1-43 and electrically stimulated, causing the dye to be endocytosed through vesicle recycling. FM1-43 was then removed from the extracellular solution, causing it to wash out from the plasma membrane but remain in the protected endocytosed vesicles. Cultures were then stimulated a second time, causing exocytosis and dye release from the labeled vesicles. Aravanis et al. found that upon fusion only a portion of the dye was released from an individual vesicle, and further, another quick electrical stimulation caused slightly more dye release. However if the latency between the two shocks was more than 23 seconds, further release was not seen. These results supported a mechanism slower than the kiss-and-run model (too fast for dye to leave vesicles), but faster than the complete fusion model seen in the frog NMJ.
Gandhi and Stevens used a pH sensitive GFP, pHluorin, fused to the intravesicular domain of synaptobrevin, to monitor vesicle recycling. This fusion protein, synaptopHluorin, fluoresces at high pHs, such as in the synaptic cleft. At low pHs, such as in synaptic vesicles, fluorescence is decreased. Monitoring the timing of fluorescence they concluded there are three modes of vesicle recycling. The first two are similar involving a vesicle that does not collapse and a fusion pore that remains open for less than a second (kiss-and-run) or 8-21 seconds (compensatory). They termed the third and longest (45 seconds) mechanism the stranded mode. In the stranded model, the vesicle membrane incorporates into plasma membrane, but cannot be endocytosed until another action potential causes an additional necessary increase in calcium.
Which Method is Dominant?
Based on these studies, a kiss-and-run mechanism seemed to dominate vesicle recycling in the mammalian central nervous system. Recently, however, Granseth et al. demonstrated the classical clathrin-mediated mechanism predominates in the hippocampus (6). Using a similar technique as Gandhi and Stevens, Granseth et al. designed sypHy by fusing pHluorin to synaptophysin, another vesicle membrane protein. Using sypHy they found a time constant of 15 seconds for endocytosis. To determine if clathrin is necessary for vesicle recycling, they disrupted the classical pathway by two methods, RNAi knockdown of the clathrin heavy chain and overexpression of a dominant-negative form of the clathrin-adaptor protein, AP180. The resulting complete blockage of endocytosis supported their hypothesis that the clathrin mediated mechanism predominately functions in hippocampal cultures.
Currently, the most convincing argument is that the both methods are utilized to different degrees depending on the stimulation frequency. While kiss-and-run recycling is utilized for high-frequency signals due to the quick recycle rate, full-collapse fusion is better suited for low-frequency signals. Therefore, in order to maintain signaling flexibility, a combination of the two methods provides a broad frequency response that can be sustained over longer time intervals (10). This ability for both mechanisms to be active was demonstrated by Aravnis et al using fluorescent marker FM1-43 to examine rat hippocampal neurons (4).
REFERENCES
1. Bittner,G.D. & Kennedy,D. Quantitative aspects of transmitter release. J. Cell Biol. 47, 585-592 (1970).
2. Heuser,J.E. & Reese,T.S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J. Cell Biol. 57, 315-344 (1973).
3. Ceccarelli,B., Hurlbut,W.P. & Mauro,A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J. Cell Biol. 57, 499-524 (1973).
4. Aravanis,A.M., Pyle,J.L. & Tsien,R.W. Single synaptic vesicles fusing transiently and successively without loss of identity. Nature 423, 643-647 (2003).
5. Gandhi,S.P. & Stevens,C.F. Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature 423, 607-613 (2003).
6. Granseth,B., Odermatt,B., Royle,S.J. & Lagnado,L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron 51, 773-786 (2006).
7. Borst J, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature 383, 431-34 (1996).
8. Katz B. The Release of Neural Transmitter Substances. Liverpool: Liverpool Univ. Press (1969).
9. Meinrenken CJ, Borst J, Sakmann B. Calcium secretion coupling at calyx of Held governed by non-uniform channel-vesicle topography. J. Neurosci. 22, 1648-67 (2002).
10. Harata NC, Aravanis AM, Tsien RW. Kiss-and-run and full-collapse fusion as modes of exo-endocytosis in neurosecretion. J. Neurochem. 97, 1546–1570 (2006).
Recent updates to the site:
List of abbreviations:
- N
- This edit created a new page (also see list of new pages)
- m
- This is a minor edit
- b
- This edit was performed by a bot
- (±123)
- The page size changed by this number of bytes
28 February 2026
|
|
02:18 | Hu 2 changes history +71 [Hugangqing (2×)] | |||
|
|
02:18 (cur | prev) −8 Hugangqing talk contribs | ||||
|
|
02:17 (cur | prev) +79 Hugangqing talk contribs | ||||
26 February 2026
| 01:16 | CHIP:Research diffhist +274 Gabor Balazsi talk contribs | ||||
| 01:13 | CHIP:Talks diffhist +1 Gabor Balazsi talk contribs | ||||
24 February 2026
|
|
08:52 | WAKNA:Basics 3 changes history +283 [Berthold Drexler (3×)] | |||
|
|
08:52 (cur | prev) +100 Berthold Drexler talk contribs (→Hier finden Sie Literatur für Einsteiger:innen in das Gebiet der Neuroanästhesie) | ||||
|
|
08:51 (cur | prev) +4 Berthold Drexler talk contribs (→Schädelhirntrauma) | ||||
|
|
08:51 (cur | prev) +179 Berthold Drexler talk contribs (→Schädelhirntrauma) | ||||
23 February 2026
|
|
m 07:41 | Gerber:Publications 2 changes history +38 [Andre P. Gerber (2×)] | |||
| m |
|
07:41 (cur | prev) 0 Andre P. Gerber talk contribs | |||
| m |
|
07:41 (cur | prev) +38 Andre P. Gerber talk contribs | |||