Agarose gel loading buffer
Loading dye is mixed with DNA samples for use in agarose gel electrophoresis. It generally contains a dye to assess how "fast" your gel is running and a reagent to render your samples denser than the running buffer (so that the samples sink in the well).
Material
Density
- Ficoll
- sucrose
- glycerol
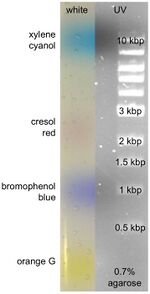
Colour
| dye | 0.5-1.5% agarose | 2.0-3.0% agarose* | CAS number | order # example |
|---|---|---|---|---|
| Xylene cyanol | 10'000-4000 bp | 750-200 bp | 2650-17-1 | Sigma X4126 |
| Cresol Red | 2000-1000 bp | 200-125 bp | 62625-29-0 | Sigma 114480 |
| Bromophenol blue | 500-400 bp | 150-50 bp | Sigma B8026 | |
| Orange G | <100 bp | ? | Sigma O3756 | |
| Tartrazine | <20 bp | <20 bp | 1934-21-0 |
*sieving agarose
Recipes for loading buffers
The precise amount of dye is not important. However, it is crucial that you prevent overlay of dye and expected DNA size. For example, if you are expecting a genotyping band of 200-400nt, you shouldn't use bromophenol blue since it will obscure your product. In this case, you should use a larger dye like xylene cyanol.
Ficoll & Orange G (6×)
- 1.5g Ficoll 400
- Orange G dye
- dH2O to 10mL
Add very small amounts of Orange G dye such that the loading dye is dark orange. Store in small aliquots at 4°C (room temperature is okay too). To use, add and mix 1/5th volume of loading dye to DNA solutions prior to loading into the wells of gels.
Sucrose & xylene cyanol / bromophenol blue (6×)
- 4g sucrose
- 25mg bromophenol blue or xylene cyanol (0.25%)
- dH2O to 10mL
Add appropriate amount to DNA sample, e.g. 5µl to 25µl.
Store at 4°C to avoid mould growing in the sucrose. 10mL of loading buffer will last for years.
Glycerol & bromophenol blue (6×)
- 3ml glycerol (30%)
- 25mg bromophenol blue (0.25%)
- dH2O to 10mL
compare CSH protocols [1] (restricted access)
NEB Loading Dye
Used by Dueber Lab (UC Berkeley). –Shyam Bhakta
Ficoll®-400 results in brighter and tighter bands when compared to glycerol loading dyes. SDS creates sharper bands, as some restriction enzymes are known to remain bound to DNA following cleavage. EDTA chelates magnesium (up to 10 mM) in enzymatic reactions, thereby stopping the reaction. Tris-HCl buffers the sample at a pH safe for DNA. In 1% agarose gels, orange G comigrates with a ~50 bp fragment, bromophenol blue with a ~300 bp, and xylene cyanol with a ~4,000 bp fragment. This 6× loading dye recipe is identical to that of NEB loading dyes, except for the addition of both xylene cyanol and Orange G (slightly reduced from 0.15%) with bromophenol blue. The Dueber Lab also adds 6× GelGreen DNA stain to the loading dye, so as not to have to pre/post-stain the gel.
| 6× | 1× (reference) | |
|---|---|---|
| per mL | ||
| Either 60% v/v Glycerol | 0.6 mL 100% glycerol | 10% Glycerol |
| or 15% m/v Ficoll®-400 | 150 mg | 2.5% Ficoll®-400 |
| 20 mM Tris-HCl, pH 8.0 | 20 mM÷cstock | 3.33 mM Tris-HCl, pH 8.0 |
| 60 mM EDTA | 60 mM÷cstock | 10 mM EDTA |
| 0.48% m/v SDS | 0.48%÷cstock. Or 4.8 mg/mL. | 0.08% SDS |
| 0.03% m/v Xylene Cyanol | 0.3 mg | 0.005% Xylene Cyanol |
| 0.03% m/v Bromophenol Blue | 0.3 mg | 0.005% Bromophenol Blue |
| 0.12% m/v Orange G | 1.2 mg/mL | 0.02% Orange G |
Specific recipes
Links
OWW
External
- Methodsbook.net: xylene or bromophenol + sucrose DNA loading buffers

- Bioron Company page on bromophenol + xylene + Ficoll DNA loading buffer
