3D Paper Microfluidics - Peiyao Zhao, Adaline Nuesse, Lucas Rozanski
Introduction
As a core of point-of-care (POC) diagnostics, microfluidics paper-based analytical devices (μPADs) are crucial for preventing the spread of infectious diseases and the improvement of global healthcare by lowering the cost and improving the feasibility of implementing such technologies in remote locations and developing regions. Current μPADs are low cost, require small amounts of sample volume and can be used easily without requiring intense training and standard lab instruments.[1] Monolayer paper devices, typically being used in lateral flow assays and spot tests,[2] have considerable drawbacks from their low flow rate and inability to perform complex processes, such as controlling the vertical and horizontal flow without them inter-mixing.[3] Thus, multilayer paperfluidics, a term which describes the expansion of a monolayer paper microfluidics device into a complex 3D structure, have been developed to circumvent those limitations and improve the capability of μPADs to meet the World Health Organization’s ASSURED criteria (affordable, sensitive, specific, user-friendly, rapid, robust, equipment-free, and deliverable) for the portable diagnostics and wearable monitoring.[4]
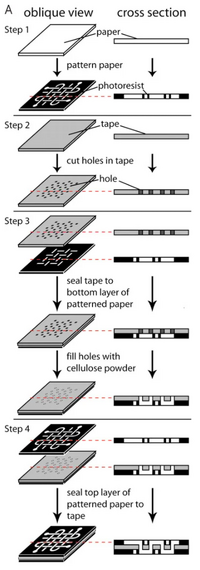
Typical 3D μPADs structures involve single-sheet 3D, stacked 3D, folding-based 3D, or slip-based 3D arrangement. The first 3D paper-based microfluidics was a stacked μPAD, which can be attributed to the Whitesides group in 2008. It was fabricated by adhering each layer to the next with double-sided tape, shown in Figure 1.[5] The double-sided tape used is water impermeable, allowing for the separation of channels between each layer of paper. To allow for vertical flow in such a device, holes can be cut into the tape to connect the channels of each layer. To complete the connection between the two layers, cellulose powder can be filled in before adhering to the top layer. This method proved to some to be too imprecise, as the assembly of the device requires both precision cutting and layering. In response, research has been conducted into alternative layering methods.
Some devices take advantage of the multiple layers by employing a folding technique, wherein adherence is achieved via magnetic sheets in the device.[6] The magnetic sheets are made of plastic, and also have holes cut out of them to allow for vertical flow. This hole can be similarly backfilled with cellulose powder or another desired flow medium. This provides the further advantage of allowing for each individual layer to be opened for analysis.
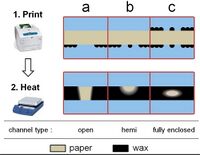
Another alternative method is to create a 3D microchannel on a single piece of paper using wax patterning, wherein wax is strategically melted into paper to create channels. This has the advantage of decreasing the risk of contamination, and also takes advantage of the properties of wax. Wax is hydrophobic, and thus, this patterning method can be used to create specific hydrophilic channels of unwaxed paper. The wax is melted into the paper in this method, and channel dimensions can be manipulated by strategically choosing the quantity and location of molten wax, as shown in Figure 2.[7]
Given the great importance and potential of 3D paperfluidics for the healthcare and medical industry, we elaborate on their design, manufacturing technique, and potential in biological analysis below, and will elaborate on the colorimetric and electrochemical detection via 3D μPAD.
Fabrication
3D µPADs are superior to 2D paperfluidics due to their ability to include multiple reactions or process steps in a compact space. Since there is now the ability to flow in the vertical direction, as well as laterally, the process speed and efficiency can be increased in a single chip by increasing the number of flow paths and increasing the functions within the layers. Since 3D µPADs build upon the concepts of 2D paperfluidics, the fabrication techniques for 2D are enhanced for 3D purposes. The two main methods for 3D µPAD fabrication are stacking and origami.[8]
Stacking
The process of stacking can be implemented by creating multiple paper layers and then connecting them together through the use of double sided adhesive tape. The adhesive tape is lined up between each layer to connect the paper without blocking the passages for fluid flow.[8] The connecting tape can be cut using a laser cutter to create channels that would be filled with cellulose powder to link the fluid routes creating multiple hydrophilic paths. The cellulose powder is necessary to fill the gap that was created by the tape to induce flow between the paper layers. In this method of adhesive stacking it has been found that the paper and tape can be difficult to align and therefore other stacking methods have been investigated.
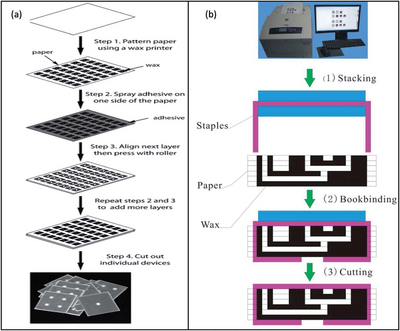
An alternative stacking method combined the technique of wax printing and an adhesive spray. In this method 2D µPADs are created using wax printing, but instead of aligning a tape to the material, an adhesive spray is layered onto the individual paper layers and pressed together. This removed the challenge of lining up the paper layers to the adhesive tape perfectly, since the spray was applied evenly and completely to the surface.[8]
Additionally, a method of stapling wax printed layers together was used to form 3D µPADs. This alternative, also called bookbinding, removed the need for an adhesive substance altogether. It was found that although this method did not align the layers perfectly, it was useful for mass fabrication of 3D µPADs. This is due to the quick mechanical process of connecting the paper layers.[8]
Origami
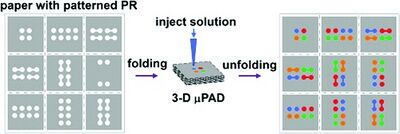
While stacking was a reasonable method for creating the 3D µPADs, origami method is beneficial because it does not need any adhesive at all. This removes any chance of additional contamination and need for laser cutting.[9] The layers have the desired patterns printed into the paper using photolithography with indicators for where the paper should be folded. The patterns control the flow of the fluid to avoid leaking, without additional energy needed.[10] The folds were held into place using a clamp or weight to keep the material in place.[8]
This technique is beneficial because it does not need additional materials like adhesive tape or cellulose powder. The contact between the channels is made only through folding and pressing of the paper. The origami folding methods can be completed in a short time span without needing extensive tools to hold it in place. Additionally, after the origami paper configuration has been used, it can be deconstructed and analyzed. The paths the different fluids traveled can be identified and analyzed for proper mixing and flow travel.[9]
Applications
The need for complex 3D microenvironments is growing rapidly. 3D µPADs allow for more assays to be analyzed within the same given area than would be on 2D µPADs. Additionally, the movement within three dimensions opens up more options for steps within a given device. There are more dimensions that occupy a smaller volume, allowing for less fluid in contact with the paper channels and therefore less material absorbed. This is an important advantage when dealing with biological samples that might be limited in sample size.[11]
Cell Culture Analysis
3D µPADs are extremely useful when creating microdivices to simulate different situations for cell cultures. Due to paper-based devices being biocompatible, the cell cultures can thrive in these conditions, allowing for different small molecules to be targeted and analyzed. For example, small molecules like glucose and lactate can be characterized by their concentrations over varying conditions.[12]
The 3D µPADs allow multiple layers to be utilized. For cell cultures analyzing concentrations of glucose and lactate, three unique layers can be identified which each layer being separated by adhesive tape, a hydrophobic layer, and a channel. Alternative cell culturing techniques analyze the lung tumor cells to notice the metabolic activity that occurs during radiation treatment. By creating 3D µPADs to analyze the tumor cells in a micro simulation, the growth of these cells after radiation can be characterized over different time spans and conditions allowing for greater understanding of the cells behavior.[12]
Cell Migration
A 3D environment is useful for analyzing how cells would behave when disturbed. 3D µPADs are used to monitor the movement of cells within the three spatial dimensions, collecting more applicable findings than if analyzed in a 2D setting. This simulation is observed when analyzing breast carcinoma cells through the addition of epidermal growth factors (EGFs) and cancer- associated fibroblasts (CAFs). Through altering EGFs and CAFs in a varying chemical gradient the behavior of the original cells is motivated into observable directions. These gradients in conjunction with a microenvironment allows for a controlled area to observe how cells would potentially react in a human body. By allowing a 3D environment, the variables can be precisely controlled with known values to allow for confident results to be determined and applied in a larger scale situation.[13]
Malaria Diagnosis
In countries where malaria is a prominent cause of death, immediate diagnosis is necessary to receive treatment. Ordinarily, to identify whether a patient has malaria, a blood sample must go through a series of laboratory examinations and tests to obtain working results. Due to the locations where malaria is prominent, typical laboratory equipment and trained technicians are not available, so an affordable diagnosis alternative is desired. A multiple layered 3D device was created by Deraney to detect any combinations of malaria. This paper-based device was created for the sample to be inputed, stored, tested, separated, and analyzed. Once the samples qualities were determined, the device would display specific colors to inform the technician of the negative or positive results. This is a easy to use and cost effective method of malaria testing in developing countries.[14]
Assays on multilayered paper based analytical devices
When used in the bioanalysis, the devices are subject to the fluid, which is then run through a varying degree of channels. The four primary detection techniques are colorimetric, electrochemical, chemiluminescence, and electro-chemiluminescence detection. This section will elaborate on the colorimetric and electrochemical detection methods.
Colorimetric Detection
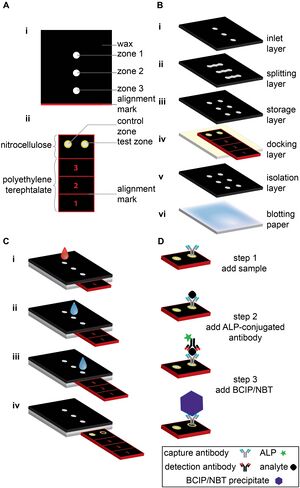
Colorimetric detection methods are among the most appealing methods due to their non-invasiveness, simplicity, fast and naked-eye detection results. Additionally, inexpensive and on-site detection can be achieved when paper-based colorimetric devices are coupled with imaging devices. When a specific reagent is applied on the surface of the sensor, the color intensity developed in the sensing area can be correlated with the analytic concentration via naked-eye detection or camera imaging.[15] Fang Li et al., reported a double-layered μPAD composed of top detection layer and button auxiliary layer, shown in Figure 5.[16] The top detection layer was modified with various colorimetric reagents for the detection of biomolecules (glucose, uric acid, lactate, and choline). The central sampling zone, microfluidic channel and detection zone are patterned via wax-screen-printing method. The auxiliary layer prevents the reagent solution from flowing backward from the detection zone to the microfluidic channel, thus enhancing the color uniformity and detection accuracy.
Furthermore, three-dimensional microfluidics devices can be distributed in different layers along with different chemical reagents to make hundreds of detection events in a very short period of time. Gomez et al., reported a well-based platform for the colorimetric measurement of glucose. This platform consists of three laminate layers, five paper layers with wax-printed barriers, and two paper chip layers. The first layer acts as the inlet for introducing the analyte solution. The glucose oxidase together with the encapsulating materials comprised of solid metal-organic framework of Zr-PCN-222(Fe) are spotted between the chip layers, which will then react with the glucose and cause the oxidation of the color reagent KI, leading to the color development. It was shown that the proposed colorimetric device exhibits a detection limit of around 0.25 mM.[17]
Another advantage of multilayered paper microfluidics is that it can perform complex assays in one single and portable device. This feature is very important to develop an enzyme-linked immunosorbent assay (ELISAs), which require mixing of multiplex reagents and removal of excess chemical reagents in succession. Whitesides et al., presented a multilayer sliding μPAD for the detection of a biomarker callled C-reactive protein (CRP) with a colorimetric ELISA. As shown in Figure 5, this microfluidic device contains a sliding strip with an active region and a stationary structure surrounding the sliding slip to store the reagents and circulate the fluid. The detection was performed by injecting the sample into the inlet and moving the strip to the inlet for subsequent assay. The resulting color in the sensing area can be analyzed visually or using any imaging device to obtain the quantification result of the biomarker’s concentration. The device achieved a detection range of 1–100 ng/mL for CRP and can be manufactured with a cost of ~ $0.50 per device on a large scale.[18]
Electrochemical Detection
Electrochemical detection, as another attractive method for the point-of-care device, has many advantages such as robustness, quantitative, high accuracy, and high sensitivity. Compared with the colorimetric sensor device, it can provide a more sensitive result. The working principle of the μPAD-based electrochemical device is based on the electric currents change from the specific interaction between bio-recognition center and target analyte via covalent and non-covalent method. Cai et al., fabricated a paper and adhesive tape based 3D μPAD combined with smartphone and electrochemical detector wirelessly for the detection of neuron-specific enolase (NSE).[19] The paper device contained two separated filter paper assembled with double-sided adhesive tape. On each paper, there are two wax-printed circles for sample area and auxiliary area for screen-printed electrodes. This device can achieve the on-site testing of NSE with a linearity from 1 to 500 ng/mL and limit of detection of 10 pg/mL.
Furthermore, μPAD-based electrochemical device have been developed for the real-time continuous monitoring of sweat biomarkers such as electrolyte (sodium, potassium, chloride, bicarbonate ions, and etc.), metabolites (glucose, lactate, etc.), biomolecules (nucleic acids, lipids, carbohydrates, and etc.).[20] Real-time measurement of biomarkers in human biofluids is critical for informing people about the emerging diseases and problems, such as stroke, diabetes, cardiovascular diseases, and etc. Li et al., demonstrated an efficient wearable electrochemical system for the simultaneous detection of glucose and lactate on a multilayer paper device,[[1]] which is illustrated in Figure 6. The first layer absorbs the sweat, the second layer includes counter and reference electrodes, the third layer contains working electrodes and the last layer is used to promote sweat evaporation. Just by simply folding the paper to connect all the hydrophilic layers, they are able to create a diffusion pathway vertically along the paper which facilitates the sweat collection, transmission, and evaporation, achieving high sensing performance and on-body thermal management.
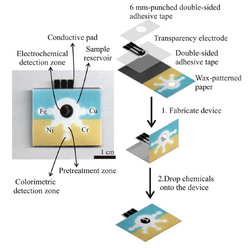
Given the simplicity of colorimetric detection and high sensitivity of electrochemical measurement, multiplexed platform combined electrochemistry and colorimetry has been developed to achieve a multidimensional analysis and higher accuracy and functionality. Henry et al., reported a three-dimensional microfluidics paper-based device for metal detection where one layer is used for multiplexed colorimetric detection of Ni, Fe, Cu, and Cr while the other layer is used for electrochemical monitoring of Pb and Cd. This device is made from paper and adhesive tape. The first layer of paper was wax-printed to define the hydrophilic channels and hydrophobic barrier for sample introduction and colorimetric detection while the second layer of paper was loaded with a screen-printed electrode on polyester film for electrochemistry measurement. By separating those two layers, they were able to improve the sensitivity and selectivity of the metal detection.[15]
Conclusions
In summary, expanding 1D and 2D paper devices to a 3D structure allows for more complex device designs and accelerates the fluid flow rate, which allows their capabilities to be enlarged. Besides, the addition of vertical flow is able to reduce the size of such devices, and thus improve their accessibility. As such, the analysis of real-world complications samples should be performed for more advanced device design. Further studies on surface modification, mixing, splitting. valving , and flow rate controlling are essential for future development of μPAD.
References
[1] de Oliveira, R. A., Camargo, F., Pesquero, N. C., & Faria, R. C. A simple method to produce 2D and 3D microfluidic paper-based analytical devices for clinical analysis. Anal. Chim. Acta, 2017, 957, 40-46. https://doi.org/10.1016/j.aca.2017.01.002
[2] Yang, Y., Noviana, E., Nguyen, M. P., Geiss, B. J., Dandy, D. S., & Henry, C. S. Paper-based microfluidic devices: emerging themes and applications. Anal. Chem.,2017, 89(1), 71-91. https://doi.org/10.1021/acs.analchem.6b04581
[3] Channon, R. B., Nguyen, M. P., Henry, C. S., & Dandy, D. S. Multilayered microfluidic paper-based devices: characterization, modeling, and perspectives. Anal. Chem.,2019, 91(14), 8966-8972. https://doi.org/10.1021/acs.analchem.9b01112
[4] Wu, G., & Zaman, M. H. Low-cost tools for diagnosing and monitoring HIV infection in low-resource settings. Bull. World Health Organ., 2012, 90, 914-920. https://doi.org/10.2471/BLT.12.102780
[5] Martinez, A. W., Phillips, S. T., & Whitesides, G. M. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proceedings of the National Academy of Sciences, 2008, 105(50), 19606-19611. https://doi.org/10.1073/pnas.0810903105
[6] Deng, H., Zhou, X., Liu, Q., Li, B., Liu, H., Huang, R., & Xing, D. Paperfluidic chip device for small RNA extraction, amplification, and multiplexed analysis. ACS Appl. Mater. Interfaces, 2017, 9(47), 41151-41158. https://doi.org/10.1021/acsami.7b12637
[7] Renault, C., Koehne, J., Ricco, A. J., & Crooks, R. M. Three-dimensional wax patterning of paper fluidic devices. Langmuir, 2014, 30(23), 7030-7036. https://doi.org/10.1021/la501212b
[8] He, Y., Wu, Y., Fu, J. Z., & Wu, W. B. Fabrication of paper-based microfluidic analysis devices: A review. RSC Adv., 2015, 5(95), 78109-78127. https://doi.org/10.1039/C5RA09188H
[9] Liu, H., & Crooks, R. M. Three-dimensional paper microfluidic devices assembled using the principles of origami. J. Am. Chem. Soc., 2011, 133(44), 17564-17566. https://doi.org/10.1021/ja2071779
[10] Sechi, D., Greer, B., Johnson, J., & Hashemi, N. Three-dimensional paper-based microfluidic device for assays of protein and glucose in urine. Anal. Chem., 2013, 85(22), 10733-10737. https://doi.org/10.1021/ac4014868
[11] Yetisen, A. K., Akram, M. S., & Lowe, C. R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip, 2013, 13(12), 2210-2251. https://doi.org/10.1039/C3LC50169H
[12] Tribhuwan Singh, A., Lantigua, D., Meka, A., Taing, S., Pandher, M., & Camci-Unal, G. Paper-based sensors: Emerging themes and applications. Sensors, 2018, 18, 2838. https://doi.org/10.3390/s18092838
[13] Campbell, J. M., Balhoff, J. B., Landwehr, G. M., Rahman, S. M., Vaithiyanathan, M., & Melvin, A. T. Microfluidic and paper-based devices for disease detection and diagnostic research. Int. J. Mol. Sci., 2018, 19(9), 2731. https://doi.org/10.3390/ijms19092731
[14] Deraney, R.N., Mace, C.R., Rolland, J.P., & Schonhorn, J.E. Multiplexed, patterned-paper immunoassay for detection of malaria and dengue fever. Anal. Chem., 2016, 88(12), 6161-6165. https://doi.org/10.1021/acs.analchem.6b00854
[15] Rattanarat, P., Dungchai, W., Cate, D., Volckens, J., Chailapakul, O., & Henry, C. S. Multilayer paper-based device for colorimetric and electrochemical quantification of metals. Anal. Chem., 2014, 86(7), 3555-3562. https://doi.org/10.1021/ac5000224
[16] Li, F., Wang, X., Liu, J., Hu, Y., & He, J. Double-layered microfluidic paper-based device with multiple colorimetric indicators for multiplexed detection of biomolecules. Sens. Actuators B Chem., 2019, 288, 266-273. https://doi.org/10.1016/j.snb.2019.02.116
[17] Ilacas, G. C., Basa, A., Nelms, K. J., Sosa, J. D., Liu, Y., & Gomez, F. A. Paper-based microfluidic devices for glucose assays employing a metal-organic framework (MOF). Anal. Chim. Acta, 2019, 1055, 74-80. https://doi.org/10.1016/j.aca.2019.01.009
[18] Verma, M. S., Tsaloglou, M. N., Sisley, T., Christodouleas, D., Chen, A., Milette, J., & Whitesides, G. M. Sliding-strip microfluidic device enables ELISA on paper. Biosens. Bioelectron., 2018, 99, 77-84. https://doi.org/10.1016/j.bios.2017.07.034
[19] Fan, Y., Liu, J., Wang, Y., Luo, J., Xu, H., Xu, S., & Cai, X. A wireless point-of-care testing system for the detection of neuron-specific enolase with microfluidic paper-based analytical devices. Biosens. Bioelectron., 2017, 95, 60-66. https://doi.org/10.1016/j.bios.2017.04.003
[20] Weber, J., Kumar, A., Kumar, A., & Bhansali, S. Novel lactate and pH biosensor for skin and sweat analysis based on single walled carbon nanotubes. Sens. Actuators B Chem.,2006, 117(1), 308-313. https://doi.org/10.1016/j.snb.2005.12.025
[21] Li, M., Wang, L., Liu, R., Li, J., Zhang, Q., Shi, G., Li, Y., Hou, C., & Wang, H. A highly integrated sensing paper for wearable electrochemical sweat analysis. Biosens. Bioelectron., 2021, 174, 112828. https://doi.org/10.1016/j.bios.2020.112828
