20.109(F14): Mod 2 Day 7 Readout of Protein
Readout Protein Abundance
Introduction
Antibodies are useful tools in the lab. Today you will use antibodies to detect a protein on a blot. This technique, called Western analysis, can give you information about the size and concentration of the protein in the pool that was separated by SDS-PAGE. In your case today, you will use a Western to identify the expression of the light sensing protein, Cph8, and the mutant versions of it. In general, detection depends on which antibody you choose, and the quality of your results depends largely on the quality of that antibody.
For Western analysis, a high quality antibody can have a relatively low affinity for its target protein. This is because the target is localized and concentrated on a blot, allowing the antibody to bind using both antibody “arms” thereby strengthening the association. Even an antibody that is loosely bound to the blot under these circumstances may dissociate then re-associate quickly since the local concentration of the target protein is high. The lower limit for protein detection is approximately 1 ng/lane, a value that varies with the size of the protein to be detected and the Western blotting apparatus that is used. For most acrylamide gels, the protein capacity for each lane is usually 100 to 200 ug (that would be 20 ul of a 5-10 ug/ul protein preparation). Thus 1 ng represents a protein that is approximately 0.001-0.002% of the total cellular protein (1 ng out of 100,000-200,000 ng). Obviously proteins that make up a more significant fraction of the total protein population will be easier to detect.
Many species can be used to raise antibodies. Most commonly mice, rabbits, and goats are immunized, but other animals like sheep, chickens, rats and even humans can be used. The protein used to raise an antibody is called the antigen and the portion of the antigen that is recognized by an antibody is called the epitope. Each antibody can recognize only a small portion of its antigen, typically 5 to 6 amino acids. Some antibodies are monoclonal, or more appropriately “monospecific,” and recognize one epitope, while other antibodies, called polyclonal antibodies, are in fact antibody pools that recognize multiple epitopes.
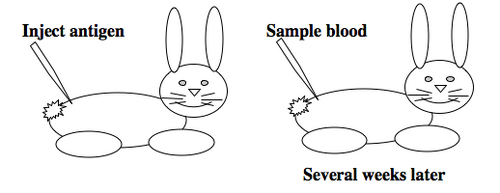
To raise polyclonal antibodies, the antigen of interest is first purified and then injected into an animal. To elicit and enhance the animal’s immunogenic response, the antigen is often injected multiple times over several weeks in the presence of an immune-boosting compound called adjuvant. After some time, usually 4 to 8 weeks, samples of the animal’s blood are collected and the cellular fraction is removed by centrifugation. What is left, called the serum, can then be tested in the lab for the presence of specific antibodies. Even the very best antisera have no more than 10% of their antibodies directed against a particular antigen. The quality of any antiserum is judged by its purity (that it has few other antibodies), its specificity (that it recognizes the antigen and not other spurious proteins) and its concentration (sometimes called its titer). Animals with strong responses to an antigen can be boosted with the antigen and then bled many times, so large volumes of antisera can be produced. However animals have limited life-spans and even the largest volumes of antiserum will eventually run out, requiring a new animal for immunization. The purity, specificity and titer of the new antiserum will likely differ from that of the first batch. High titer antisera against bacterial and viral proteins can be particularly precious since these antibodies are difficult to raise; most animals have seen these immunogens before and therefore don’t mount a major immune response when immunized. Antibodies against toxic proteins are also challenging to produce if they make the animals sick.
We will be using a polyclonal antibody raised against a soluble form of EnvZ to which six histidine residues were fused. This His6-EnvZ fusion protein could be purified based on the affinity of the histidine residues for nickel. In fact, the protein was purified during IAP 2010 by a former 20.109 student, Helen Chen. Thank you, Helen! The pool of protein was then sent to a company called Covance where it was injected into a bunny and the bunny's serum was harvested periodically.
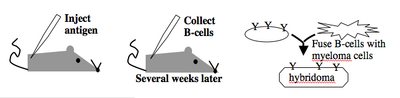
Monoclonal antibodies overcome many limitations of polyclonal pools in that they are specific to a particular epitope and can be produced in unlimited quantities. However, more time is required to establish these antibody-producing cells, called hybridomas, and it is a more expensive endeavor. Antibody-secreting cells are first isolated from an immunized animal, usually a mouse, and then fused with an immortalized cell line such as a myeloma. The fusion can be accomplished by incubating the cells with polyethylene glycol (antifreeze), which facilitates the joining of the plasma membranes of the two cell types. A fused cell with two nuclei can be resolved into a stable hybridoma after mitosis. The unfused antibody-secreting cells have a limited lifespan and so die out of the hybridoma population, but the myelomas must be removed with some selection against the unfused cells. Production of stable hybridomas is tedious and difficult but often worth the effort since monoclonal antibodies can recognize covalently-modified epitopes specifically. These are invaluable for experimentally distinguishing the phosphorylated or glycosylated forms of an antigen from the unmodified forms.
Making antibodies is big business since they can be useful therapeutics. Whereas the 2002 market for monoclonal therapeutic antibodies was estimated at almost $300 million, sales grew to $43 billion in 2010 and are predicted to reach nearly $58 billion in 2016 link. Successful antibody treatments, however, require clever engineering discoveries to “humanize” antibodies raised in other animals, as well as speedier development, well-protected patents, improvements in drug-delivery methods and cost efficient production of the therapeutics.
Protocols
Probe Western blot
- You should retrieve the blot that you made last time and pour 10 ml of the TBS-T + milk solution into a 15 ml conical tube. The rest of the TBS-T + milk can be discarded down the sink.
- Wear gloves and cut the blot next to the markers in the middle of the blot (if it hasn't been cut last time).
- Place the blot lanes 1-5 in one blotting container, and the other portion of the blot (lanes 6-10) in another container.
- To the 10 ml of TBS-T + milk that you saved in the 15 ml conical tube, add 10 ul of anti-H6EnvZ antibody.
- Add the diluted primary antibody to your blot.
- Cover the containers, label with your team color, and place on the platform shaker that's in the chemical hood for 45 minutes. During this time you can work on your report, ask questions of each other or the teaching faculty, and work on your oral presentation if that's coming up too.
- Pour the antibody solution into a conical tube, writing the identity of the antibody and the date on the tube.
- Give the blots a quick rinse with TBS-T (no milk), enough to cover the blot (volume is not critical here).
- Wash the blot on the platform shaker 2 times with TBS-T at room temperature, five minutes per wash. Again the volume of the wash solution is not critical.
- Add secondary antibody (1:1,000 Goat-antirabbit-alkaline phosphatase) in 15 ml TBS-T and incubate on the platform shaker at room temperature for 30 minutes.
- Wash the blot as before (rinse and two washes).
- When you are done washing, mix 250 ul of each of the solutions from the alkaline phosphatase substrate kit into the provided tube of 25 ml 1X developing solution.
- Add developing solution and shake on the platform shaker watching for color to develop. Rinse the blot with water when bands are evident. Recall you anticipated what size protein you are expecting for Cph8. The blots can be left overnight in developing solution or water. One of the teaching faculty will then scan the blot and post the results for you.
Additional Experiments? Questions?
There is time planned in to today's lab to run experiments of your choosing, to talk with other groups about their results, and to work on your research article.
DONE!
For Next Week (or sooner!)
- Your research article describing this work is due in just over one week. This assignment is due by 12:00 pm on Wednesday, November 12th. Please turn in your research article electronically by uploading it to the Stellar website that is associated with our class. It is important that you name your file according to this convention: Firstinitial_Lastname_LabSection_assignment.doc, for example: B_Obama_TR_ResArt.doc There will be a 1/3 letter grade penalty for each day (24 hour period) late. If you are submitting your assignment after the due date, it must be emailed to nkuldell AT mit DOT edu.
- Some of you will also be busy preparing a Journal Club presentation for next time. The slides for your presentation should be uploaded to the Stellar website that is associated with our class. The presentation order will be determined by the order that your finished slides are uploaded.
- If you have not yet contributed your thoughts, comments and ideas to the class blog, then remember your participation grade includes this work. Some ideas for your post are here.
- Once all that's in, begin to familiarize yourself with the content of the third experimental module by reading the front page for the module as well as skimming the introduction to the first day of labwork.
Reagents list
- TBS-T Tris-Buffered Saline + Tween
- polyclonal anti-H6EnvZ from Covance Research, raised in rabbits
- polyclonal antirabbit-AP from BioRad, raised in goat (changed by NK from antimouse on 11.13)
- BioRad AP detection reagents
- 1 ml 25x detection stock + 24 ml H2O with 0.25 ml solnA and 0.25 ml solnB.
Navigation Links
Next Day: Mod 2 Day 8: Journal Club II Previous Day: Mod 2 Day 6: Readout DNA